
PIASy deficiency mitigates thoracic aortic aneurysm formation via the TGF-β/Smad2/3 pathway
Highlight box
Key findings
• Protein inhibitor of activated STAT 4 (PIASy) is significantly upregulated in human and murine thoracic aortic aneurysm (TAA) tissues.
• PIASy deficiency protects against TAA formation by maintaining the contractile phenotype of vascular smooth muscle cells (VSMCs) via the transforming growth factor-β (TGF-β)/Smad2/3 pathway.
What is known, and what is new?
• VSMC phenotypic switching and TGF-β signaling are critical in the pathogenesis of TAA.
• New findings: (I) PIASy was identified as a key regulator promoting VSMC de-differentiation; (II) PIASy deficiency restores TGF-β/Smad2/3 signaling and prevents TAA.
What is the implication, and what should change now?
• Targeting PIASy may offer a novel therapeutic approach for TAA.
• Future research should focus on in vivo validation and drug development targeting PIASy.
Introduction
Thoracic aortic aneurysm (TAA) refers to an abnormal expansion of the aortic root, ascending aorta, aortic arch, or descending aorta, exceeding 50% of the normal blood vessel diameter (1,2). Its harm often lies in the occurrence of secondary aortic aneurysm rupture, aortic dissection, and severe aortic insufficiency (3). TAA has a high disability and fatality rate, and is one of the most dangerous cardiovascular diseases.
Vascular smooth muscle cells (VSMCs) comprise the majority of the cellular components in the middle aorta, which are critical regulators of blood flow and distribution, as well as blood pressure (4-6). VSMC phenotypic transformation occupies a central position in the pathogenesis of TAA. There is increasing evidence that inhibiting the switch from the contractile state, which is responsible for regulating blood flow and pressure, to a secretory phenotype, which is capable of synthesizing matrix metalloproteinase-2 and matrix metalloproteinase-9, is valuable in TAA prevention (7,8). Therefore, VSMCs have become a significant target in TAA research.
Protein inhibitor of activated STAT 4 (PIASy, also known as PIAS4) belongs to the PAIS protein family, and was found to be an inhibitor of the STAT protein family (9). PIAS proteins, which include PIAS1, PIAS3, PIASxα, PIASxβ, and PIASy (9,10), serve as essential Small ubquitin-related modifier E3 (SUMO E3) ligases that facilitate the SUMOylation of target proteins. They play a significant role in modulating the transcriptional activities of various transcription factors, highlighting their importance in cellular regulatory processes. Notably, PIASy has been previously reported to modulate transforming growth factor-β (TGF-β) signaling by SUMOylation of Smad proteins, suggesting a mechanistic link between PIASy activity and Smad2/3 pathway regulation (11,12). Previous research has shown that PIASy can facilitate myocardium SUMOylation and promote its protein stability (13-15). However, the functional role of PIASy in VSMCs, particularly in the setting of TAA, is poorly understood.
In the present study, we examined the expression of PIASy in VSMCs in TAA tissue and evaluated the effects of PIASy knockdown in vitro. Our findings revealed that the loss of PIASy modulates VSMC phenotypic switching via the regulation of the TGF-β pathway, and offer new insights into potential therapeutic strategies for TAA. We present this article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-984/rc).
Methods
Experimental animals
All the experimental procedures in this study were approved by the Ethics Committee of Renji Hospital in Shanghai, China (No. SYXK2023-0041). The procedures were conducted in accordance with the “Guidelines for the Care and Use of Laboratory Animals” issued by the National Health and Family Planning Commission of China. A protocol was prepared before the study without registration. All animal experiments were conducted at the Laboratory Animal Center of Renji Hospital (Shanghai Jiao Tong University School of Medicine), Shanghai, China. Male C57BL/6 mice, aged 8 to 10 weeks, were obtained from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences (Shanghai, China).
Human specimens
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Our collection of clinical TAA and normal aortic tissue specimens was approved by the Ethics Committee of Renji Hospital (Shanghai, China, No. 2022-33K). All the participants (or their legal guardians as necessary) provided informed consent. We collected aortic aneurysm specimens from 10 patients who underwent surgical procedures, and normal specimens from 10 additional patients who underwent extracorporeal bypass surgery at the same hospital. To ensure the highest quality, each specimen was carefully divided as follows: one part was promptly frozen in liquid nitrogen and stored at −80 ℃, while the other was fixed in 4% paraformaldehyde. All the procedures were conducted in strict accordance with the ethical guidelines set forth by the Ethics Committee to ensure the integrity of the study and the well-being of the participants.
AngII induces aorta formation
We successfully developed a TAA model by implanting apolipoprotein E (ApoE)-deficient mice with an osmotic minipump (Alzet, Model 2004, California, USA). This setup enabled the delivery of recombinant human angiotensin II (AngII) (Sigma-Aldrich, Missouri, USA) at 1,000 ng/min/kg for 4 weeks, effectively promoting aneurysm formation. The male ApoE−/− mice were randomly assigned to the following two groups: the phosphate buffered saline (PBS) group, and the AngII group. The mice in the PBS group received equal volumes of normal saline, while those in the AngII group received AngII via a subcutaneous osmotic minipump.
Histological analyses
Fresh tissue samples were meticulously fixed in 4% paraformaldehyde for 24 hours, and then dehydrated using a graded series of ethanol and xylene, after which, they were embedded in paraffin wax blocks. The tissue samples were then sectioned into 5-µm-thick slices using a paraffin microtome and mounted onto glass slides. For the immunohistochemical staining, the sections were deparaffinized in xylene, and rehydrated through a graded series of ethanol. After washing with PBS, endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide in methanol. After additional washing with PBS, antigen retrieval was performed in citrate buffer at 95 ℃. The sections were then washed with PBS, blocked with 10% goat serum for 30 minutes at room temperature, and then incubated overnight at 4 ℃ with primary antibodies: PIASy (14242-1-AP, Proteintech). The next day, after PBS washing, the sections were incubated with a biotinylated anti-rabbit secondary antibody (#8114, CST) at 37 ℃ for 30 minutes, followed by further PBS washing. A biotin-coupled horseradish peroxidase (HRP) complex was then added and incubated at 37 ℃ for 30 minutes. After further PBS washing, color development was performed using 3,3’-diaminobenzidine as a HRP substrate for 2 minutes. The sections were rinsed with tap water, counterstained with hematoxylin, dehydrated using graded ethanol and xylene, and finally sealed with neutral resin.
Culture of mouse vascular smooth muscle (MOVAS) cells and establishment of PIASy knockdown cell lines
The MOVAS mouse VSMC line (ATCC, CRL-2797) was obtained from the American Type Culture Collection (ATCC) and cultured at 37 ℃ in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum and 1% penicillin-streptomycin. PIASy-interfering RNA virus and the corresponding control virus were obtained from VectorBuilder (shPIASy#1: GTGGTGCTGAGGATCTGTTAC, shPIASy#2: TTCAACATGCTGGACGAATTG) and transduced into cells in accordance with the manufacturer’s instructions. After 48 hours of infection, puromycin (5 µg/mL) was added to the culture medium for selection, leading to the establishment of the non-targeting control shRNA (shNC) and shRNA targeting PIASy (shPIASy) cell lines. Before further experiments, the MOVAS, shNC, and shPIASy cells were treated with either PBS or AngII (100 nM) for 24 hours (16).
Western blot
The cells were lysed using Radio-Immunoprecipitation Assay buffer (RIPA) buffer from Thermo (Massachusetts, USA), enriched with Roche’s (Basel, Switzerland) protease and phosphatase inhibitors for optimal results. Next, equal amounts of cell lysates were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gels for electrophoresis and then expertly transferred onto membranes. To enhance the process, the membranes were blocked with 5% fetal bovine serum and incubated overnight at 4 ℃ with specific primary antibodies, before the ideal secondary antibodies were added. The following antibodies were used for Western blotting: PIASy (14242-1-AP, Proteintech, Wuhan, China), β-actin (60008-1-Ig, Proteintech), GAPDH (60004-1-Ig, Proteintech), PDGFRβ (ab32570, Abcam, Cambridge, United Kingdom), SPP1 (22952-1-AP, Proteintech), KLF5 (51586, CST, Massachusetts, USA), α-SMA (A5550, Selleck, Texas, USA), Calponin (A5609, Selleck), SM22 (10493-1-AP, Proteintech), Smad2/3 (AF03499, AiFang biological, Hunan, China), TGF-β1 (AFW2124, AiFang biological), anti-rabbit (#7074, CST), and anti-mouse (#7076, CST). Protein bands were visualized using the Odyssey® CLx imaging analyzer, and the densitometric analysis was performed using ImageJ. The protein expression levels were normalized to β-actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Immunofluorescence assay
After fixation with paraformaldehyde, the cells or cryosections were permeabilized using 0.3% Triton X-100, and then blocked with 5% bovine serum albumin. The samples were incubated with specific primary antibodies [KLF5 (#51586, CST), α-SMA (A5550, Selleck)] at 4 ℃ overnight. After multiple washes with PBS, the cells were incubated with fluorescently labeled secondary antibodies (#4414, CST) for 1–2 hours at room temperature. The nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) (28718-90-3, Sigma-Aldrich) for 5 minutes before imaging. Immunofluorescence signals were visualized using a confocal microscope (LSM 710, Zeiss, Oberkochen, Germany).
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the shNC and shPIASy cell lines using TRIzol reagent (Takara, Shiga, Japan) in accordance with the manufacturer’s instructions. RNA purity was assessed by measuring the OD260/OD280 ratio using a NanoDrop 2000 spectrophotometer (Thermo). Complementary DNA (cDNA) was synthesized from messenger RNA (mRNA) using the PrimeScript™ RT reagent kit (Takara). The resulting cDNA was diluted fourfold with distilled water and used for RT-qPCR, which was performed using a fluorescence-based RT-qPCR kit (Takara) on a Roche RT-qPCR instrument.
Bulk RNA sequencing (RNA-seq) and data analysis
The RNA-seq analysis of the shNC and shPIASy MOVAS cell lines was performed by Sangon Biotech Co., Ltd. (Shanghai, China). The MOVAS cell lines were treated with AngII as described above, and total RNA was extracted. cDNA was synthesized according to the Gene Expression Omnibus sequence (Geo-seq) protocol (17). Sequencing libraries were prepared with the Hieff NGS™ MaxUp Dual Mode mRNA Library Preparation Kit (Yeasen Biotechnology, Shanghai, China) and assessed using the Qubit™ ssDNA Detection Kit (Yeasen Biotechnology). Low-quality reads and subsequences were removed using Trim Galore version 0.4.4. The cleaned reads were aligned to the mm10 reference genome using Bowtie2, and the uniquely aligned reads were quantified with featureCounts. The RNA-seq data were also aligned to the GRCm39 mouse genome using HISAT2 (version 2.1.0).
Enrichment analysis of differentially expressed genes
The differential gene expression analysis of the RNA-seq data was performed using the DESeq2 package in R version 4.4.2. Genes with a P value <0.05 and a fold change >1.5 were considered significantly upregulated. The enrichment analysis of these upregulated genes was conducted using the enrichKEGG package to identify any associated pathways, and the results were visualized using ggplot2. Additionally, a gene set enrichment analysis (GSEA) of all the differentially expressed genes was performed using the ClusterProfiler package.
Statistical analysis
A comprehensive statistical analysis was conducted using GraphPad Prism version 9.4 (GraphPad Software, San Diego, CA, USA) and R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). The data are presented as the mean ± standard deviation. The Student’s t-test and an analysis of variance were used to evaluate group differences, with significance thresholds established at P<0.05, P<0.01, and P<0.001. A two-sided P value of less than 0.05 was considered statistically significant.
Results
PIASy was elevated in human TAA tissue
To explore whether PIASy was associated with TAA, its expression was quantified in aortic tissues from patients with and without TAA. Both the mRNA and protein levels of PIASy were significantly higher in the TAA tissues than the normal aortic control tissues (Figure 1A-1C). These results suggest that PIASy plays a role in TAA.

PIASy was upregulated in the AngII-induced mouse aortic aneurysm model
The role of PIASy in TAA was further investigated using an AngII-induced aneurysm model in ApoE−/− mice. The ApoE−/− mice were treated with PBS or AngII (1,000 ng/min/kg) for 28 days. The Western blot and immunohistochemistry results showed that PIASy expression was significantly increased in the aortic tissues of the model group compared to those of the sham-treated control group (Figure 2A,2B). Notably, the immunohistochemical analysis revealed that PIASy was primarily localized in the tunica media of the TAA tissues (Figure 2C,2D), which is predominantly composed of VSMCs. These results suggest that PIASy may be involved in the development of TAA via the regulation of the VSMC phenotype.

PIASy deficiency alleviated TAA formation
The VSMC phenotype switch is the core pathogenesis in TAA formation. As PIASy was specially overexpressed in the tunica media of the TAA tissue, we speculated that the silencing of PIASy would affect the VSMC phenotypic switch. The stable knockdown of PIASy was successfully achieved in the MOVAS cells using lentiviral short-hairpin RNA. We found that the protein levels of PIASy were significantly reduced in the shPIASy cells compared to the shNC control cells (Figure 3A).

To validate the effect of PIASy on VSMC differentiation in vitro, the shNC and shPIASy MOVAS cells were stimulated with AngII for 24 hours as described above. Genes, including Calponin, SM22, and α-SMA, were downregulated, while the secretory genes, including KLF5, SPP1, and PDGFRβ, were upregulated. The RT-qPCR analysis also showed that PIASy knockdown rescued the level of contractile markers and countered AngII-induced changes in secretory gene markers (Figure 3B-3G). Meanwhile, the Western blot analysis revealed that the contractile protein levels decreased and the secretory protein levels increased. The PIASy knockdown limited these changes in protein expression (Figure 3H,3I). To further verify the effect of PIASy on the VSMC phenotype switch, multiple immunofluorescence staining was performed. The staining results indicated that the absence of PIASy negatively affected VSMC differentiation (Figure 3J). These results indicate that the specific deletion of PIASy in VSMCs prevents TAA formation in vitro.
Loss of PIASy prevented aortic aneurysm through TGF-β/Smad pathway
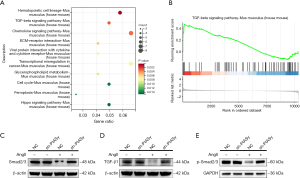
We conducted bulk RNA-seq to identify the dysregulated genes in response to the PIASy knockdown MOVAS cell line treated with AngII. The Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses indicated that the differentially expressed genes were significantly enriched in the TGF-β signaling pathway (Figure 4A). The GSEA also revealed that the TGF-β signaling pathway was significantly enriched (Figure 4B). TGF-β/Smad signaling plays a vital role in VSMC reprogramming (18,19). To gain insights into the mechanism and examine whether PIASy was involved in the TGFβ pathway in TAA formation, we performed Western blot. The Western blot analysis confirmed that the TGF-β and Smad2/3 levels were downregulated in the AngII-treated shNC cells, while PIASy knockdown rescued their expression (Figure 4C,4D). Moreover, we performed immunoblotting for phosphorylated Smad2/3 and observed that PIASy knockdown restored p-Smad2/3 levels (Figure 4E). These results suggest that the loss of PIASy prevents the transformation of VSMC from a contractile phenotype to a secretory phenotype by regulating the TGF-β/Smad signaling pathway, and ultimately prevents TAA formation.
Discussion
Current treatment of TAA primarily relies on surgical intervention, with limited pharmacological options for prevention. The identification of PIASy as a modulator of VSMC differentiation offers a potentially novel target that addresses this clinical gap. The key findings of our study indicate that a deficiency in PIASy is associated with a reduction in the risk of TAA. We found that PIASy is highly expressed in human and murine TAA tissues, which shows its importance in TAA formation. We also performed knockdown experiments in vitro, and found that suppressing PIASy inhibits the VSMC phenotype switch from a contractile phenotype to a secretory phenotype. To our knowledge, this is the first study to show that PIASy critically affects VSMC reprogramming.
The phenomenon of VSMC phenotype switching relates to the differentiation of macrophages on stimulation, resulting in the emergence of distinct cell types, including the contractile phenotype and the secretory phenotype (20,21). Contractile VSMCs have a significant effect on the vascular structure, elasticity, and homeostasis. Secretory phenotype VSMCs with high osteopontin expression have enhanced matrix metalloproteinase synthesis, and increased migratory and proliferative abilities. The phenotypic transformation of VSMCs is crucial in the pathogenesis of TAA (7,8). VSMC differentiation is affected by multiple factors, including mechanical stress and inflammation.
Through bulk RNA-seq and other in vitro experiments, we discovered that PIASy regulates the VSMC shift through the TGF-β pathway. Furthermore, our research revealed that PIASy inhibition corrected the dysregulated expression of key target genes—such as Smad3, SM22α, KLF5, and SPP1—and restored the balance of VSMC phenotypes, which collectively contributed to the prevention of TAA development.TGF-β is a multifunctional factor essential for cell proliferation, differentiation, apoptosis, etc. (22). Recent research showed that canonical TGF-β signaling plays an important role in aortic aneurysms by regulating VSMC reprogramming (23). Studies have demonstrated that VSMC-specific deletion of TGF-β signaling leads to macrophage infiltration, vascular inflammation, and spontaneous pathological remodeling of the aortic wall, all of which collectively accelerate the progression of TAA (15,24). Our findings that PIASy is an upstream regulator of TGF-β in the biology of VSMCs complement those of previous studies (15,25). We also found a novel mechanism of pathogenesis in TAA formation.
However, this study had certain limitations. First, this study showed that PIASy mediates the TGF-β pathway to exert its function; however, an in-depth and detailed analysis of the downstream needs to be conducted. Second, experiments need to be conducted with VSMC PIASy knockout mice to prove the results in vivo. PIASy’s role in modulating transcription is complex. Complete PIASy inhibition may inadvertently suppress protective anti-inflammatory TGF-β signals in certain contexts, warranting caution in translational applications.
Beyond TAA, PIASy has also been implicated in other cardiovascular processes, such as myocardial SUMOylation and potentially atherosclerosis, although direct evidence remains limited (26,27).
Conclusions
In conclusion, our findings indicate that PIASy promotes TAA development by facilitating VSMC phenotypic switching at least in part via the TGF-β/Smad axis. Targeting PIASy may thus represent a promising therapeutic avenue for preventing or halting TAA progression. However, the clinical feasibility of targeting PIASy remains to be further investigated, as no current drugs specifically modulate PIASy activity. Future studies should focus on pharmacological validation and safety assessments.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-984/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-984/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-984/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-984/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010;55:841-57. [Crossref] [PubMed]
- Ostberg NP, Zafar MA, Ziganshin BA, et al. The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective. Biomolecules 2020;10:182. [Crossref] [PubMed]
- Mussa FF, Horton JD, Moridzadeh R, et al. Acute Aortic Dissection and Intramural Hematoma: A Systematic Review. JAMA 2016;316:754-63. [Crossref] [PubMed]
- Han X, Xu S, Hu K, et al. Early growth response 1 exacerbates thoracic aortic aneurysm and dissection of mice by inducing the phenotypic switching of vascular smooth muscle cell through the activation of Krüppel-like factor 5. Acta Physiol (Oxf) 2024;240:e14237. [Crossref] [PubMed]
- Zhang F, Guo X, Xia Y, et al. An update on the phenotypic switching of vascular smooth muscle cells in the pathogenesis of atherosclerosis. Cell Mol Life Sci 2021;79:6. [Crossref] [PubMed]
- Brown IAM, Diederich L, Good ME, et al. Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension. Arterioscler Thromb Vasc Biol 2018;38:1969-85. [Crossref] [PubMed]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004;84:767-801. [Crossref] [PubMed]
- Chakraborty R, Ostriker AC, Xie Y, et al. Histone Acetyltransferases p300 and CBP Coordinate Distinct Chromatin Remodeling Programs in Vascular Smooth Muscle Plasticity. Circulation 2022;145:1720-37. [Crossref] [PubMed]
- Liu B, Liao J, Rao X, et al. Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci U S A 1998;95:10626-31. [Crossref] [PubMed]
- Chung CD, Liao J, Liu B, et al. Specific inhibition of Stat3 signal transduction by PIAS3. Science 1997;278:1803-5. [Crossref] [PubMed]
- Long J, Matsuura I, He D, et al. Repression of Smad transcriptional activity by PIASy, an inhibitor of activated STAT. Proc Natl Acad Sci U S A 2003;100:9791-6. [Crossref] [PubMed]
- Long J, Wang G, Matsuura I, et al. Activation of Smad transcriptional activity by protein inhibitor of activated STAT3 (PIAS3). Proc Natl Acad Sci U S A 2004;101:99-104. [Crossref] [PubMed]
- Liang M, Cai Z, Jiang Y, et al. SENP2 Promotes VSMC Phenotypic Switching via Myocardin De-SUMOylation. Int J Mol Sci 2022;23:12637. [Crossref] [PubMed]
- Rabellino A, Andreani C, Scaglioni PP. The Role of PIAS SUMO E3-Ligases in Cancer. Cancer Res 2017;77:1542-7. [Crossref] [PubMed]
- Cui M, Cai Z, Chu S, et al. Orphan Nuclear Receptor Nur77 Inhibits Angiotensin II-Induced Vascular Remodeling via Downregulation of β-Catenin. Hypertension 2016;67:153-62. [Crossref] [PubMed]
- Fu H, Shen QR, Zhao Y, et al. Activating α7nAChR ameliorates abdominal aortic aneurysm through inhibiting pyroptosis mediated by NLRP3 inflammasome. Acta Pharmacol Sin 2022;43:2585-95. [Crossref] [PubMed]
- Nagalakshmi U, Waern K, Snyder M. RNA-Seq: a method for comprehensive transcriptome analysis. Curr Protoc Mol Biol 2010;Chapter 4:Unit 4.11.1-13.
- Bunton TE, Biery NJ, Myers L, et al. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res 2001;88:37-43. [Crossref] [PubMed]
- Mallat Z, Ait-Oufella H, Tedgui A. The Pathogenic Transforming Growth Factor-β Overdrive Hypothesis in Aortic Aneurysms and Dissections: A Mirage? Circ Res 2017;120:1718-20. [Crossref] [PubMed]
- Li W, Li Q, Jiao Y, et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest 2014;124:755-67. [Crossref] [PubMed]
- Hu JH, Wei H, Jaffe M, et al. Postnatal Deletion of the Type II Transforming Growth Factor-β Receptor in Smooth Muscle Cells Causes Severe Aortopathy in Mice. Arterioscler Thromb Vasc Biol 2015;35:2647-56. [Crossref] [PubMed]
- Liu Y, Li M, Chen Z, et al. BRISC-Mediated PPM1B-K63 Deubiquitination and Subsequent TGF-β Pathway Activation Promote High-Fat/High-Sucrose Diet-Induced Arterial Stiffness. Circ Res 2025;136:297-314. [Crossref] [PubMed]
- Quelquejay H, Al-Rifai R, Silvestro M, et al. L-Wnk1 Deletion in Smooth Muscle Cells Causes Aortitis and Inflammatory Shift. Circ Res 2024;135:488-502. [Crossref] [PubMed]
- Wang J, Da X, Chen Y, et al. Glutamine Protects against Mouse Abdominal Aortic Aneurysm through Modulating VSMC Apoptosis and M1 Macrophage Activation. Int J Med Sci 2024;21:1414-27. [Crossref] [PubMed]
- Imoto S, Sugiyama K, Muromoto R, et al. Regulation of transforming growth factor-beta signaling by protein inhibitor of activated STAT, PIASy through Smad3. J Biol Chem 2003;278:34253-8. [Crossref] [PubMed]
- Xiao H, Zhou H, Zeng G, et al. SUMOylation targeting mitophagy in cardiovascular diseases. J Mol Med (Berl) 2022;100:1511-38. [Crossref] [PubMed]
- Takabe W, Alberts-Grill N, Jo H. Disturbed flow: p53 SUMOylation in the turnover of endothelial cells. J Cell Biol 2011;193:805-7. [Crossref] [PubMed]


