
Correlation of inflammatory markers with progression-free survival in advanced lung cancer patients treated with immune checkpoint inhibitors in combination with chemotherapy: a real-world study
Introduction
Immune checkpoint inhibitors (ICIs) have been approved worldwide in advanced lung cancers consisting of small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) with better clinical outcomes. In China, ICIs principally represented by programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors have been used in the first-line treatment of advanced lung cancer by Chinese Society of Clinical Oncology (CSCO) since 2018. Biomarkers like PD-L1 (1) and tumor mutational burden (TMB) (2) help to differentiate patients who benefited from ICI therapy (3). However, patients with lower PD-L1 expression (1–3% positive tumor cells) also can benefit more from ICI therapy after a long time in the real world. Meanwhile, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) were used in predicting clinical outcomes (4) in advanced NSCLC (5) on chemotherapy (6), neoadjuvant chemotherapy (7), and targeted therapy (8), but are not used in immunotherapy combined with platinum-based chemotherapy. Therefore, more biomarkers are designed to assess the predictive value in addition to the above markers to find more simple, easy to calculate, and accessible markers from blood routine examination for ICIs combined with platinum-based chemotherapy in advanced lung cancer in China. We present this article in accordance with the STROBE reporting checklist (9) (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2198/rc).
Methods
Patients and medicines
The retrospective analysis was performed using data from patients treated in Tianjin Medical University Cancer Institute and Hospital between January 2018 and December 2022. Patients in advanced lung cancer (IIIb–IV stage), with complete data in the clinical record and routine blood parameters, Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤2, and who had received at least two cycles of ICI combined with platinum-based chemotherapy were eligible for this analysis. Patients who had oncogene-addicted malignancies [epidermal growth factor receptor (EGFR), ROS proto-oncogene 1 receptor tyrosine kinase (ROS1), anaplastic lymphoma kinase (ALK) mutations/translocations], more than two cancers, chronic inflammatory diseases, autoimmune diseases, incomplete data in clinical records and routine blood parameters, or interstitial lung diseases were ineligible. The patient details were de-identified. Pembrolizumab, sintilimab, tislelizumab, nivolumab, and camrelizumab were used in NSCLC treatment. And durvalumab and atezolizumab were used in SCLC treatment. Platinum-based chemotherapy included etoposide + carboplatin (EC), pemetrexed disodium + carboplatin (PC), paclitaxel + carboplatin (TC), paclitaxel + cisplatin (TP) and gemcitabine + carboplatin (GC). The study was conducted according to the Declaration of Helsinki and its subsequent amendments and approved by the Ethics Committee of Tianjin Fourth Centre Hospital (No. SZXLL-2023-KY034). Tianjin Medical University Cancer Institute and Hospital was informed and agreed on the study. The informed consent was obtained from all patients.
Records collection
Age, gender, histological type, clinical stage, smoking index, PS, treatment lines, chemotherapy regimens, complete blood cell counts and lactate dehydrogenase (LDH) in patients were collected by the electronic medical record system of the hospital. Tumor size was assessed by computed tomography or positron emitting tomography every two treatment cycles. Progression-free survival (PFS) was a period determined from treatment beginning to clinical and radiographic progression or death for any cause. There were four treatment periods which were state 0 before treatment, state 1 before the second treatment, state 2 maximizing therapeutic benefits and state 3 disease progression. The maximizing therapeutic benefits referred to the period from beginning to acquiring minimum tumor size by imaging diagnosis. Inflammatory markers, NLR, MLR, eosinophil-to-lymphocyte ratio (ELR), PLR, systemic immune inflammation index (SII) (10), pan-immune inflammation value (PIV) (11), were calculated at four treatment states. Lung immune prognostic index (LIPI) and iSEND immune score (12) were calculated at state 0.
Following up
Complete remission (CR), portion remission (PR), stable disease (SD), and progression of disease (PD) were evaluated by Response Evaluation Criteria in Solid Tumors (RECIST 1.1). The end date of follow-up recorded as May 31, 2023 was the target date if patients did not progress to PD.
Statistical analysis
SPSS 21.0 software was used for statistical analysis. The median was the cut-off value in NLR, MLR, ELR, PLR, SII, PIV, LIPI, iSEND and LDH. The one-way analysis of variance (ANOVA) compared the mean of inflammatory markers at state 0, state 2 and state 3. The medically relevant features and peripheral blood inflammatory markers were variables. Kaplan-Meier analysis for PFS was performed. Hazard ratios (HR) and 95% confidence intervals (CI) with the univariate and multivariate Cox proportional hazard model were calculated. Covariates with a P value less than 0.2 from the Kaplan-Meier analysis were chosen in multivariate analysis. A P value <0.05 was statistically significant.
Results
Associations between inflammatory markers, LDH levels and therapeutic response
A series of 232 advanced lung cancer patients were finally enrolled in this study (Table S1). When the treatment response reached maximizing therapeutic benefits (state 2), ELR (P<0.001), PLR (P=0.005), SII (P<0.001), PIV (P=0.001), and LDH (P=0.005) decreased significantly compared with those before treatment (state 0) (Table S2).
Kaplan-Meier analysis for PFS
The mean time from before treatment to maximizing therapeutic benefits was 95.5 days. There were significant correlations in ELR1 (P=0.02), MLR2 (P=0.02), ELR2 (P=0.02), and LDH3 (P=0.003) with PFS, and high ELR1 (≥0.0265), high ELR2 (≥0.034), low MLR2 (<0.3699) and low LDH3 (<216) had longer PFS (Table S3, Figure 1).
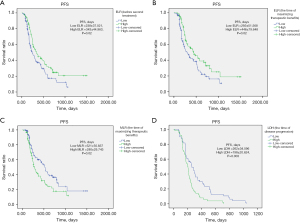
Univariate and multivariate Cox regression analyses for PFS
Univariate Cox proportional hazard regression analysis revealed that higher ELR2 (≥0.034) (ELR at maximizing therapeutic benefits) was associated with a significantly better PFS. In contrast, higher MLR2 (≥0.3699) (MLR at maximizing therapeutic benefits) and higher LDH3 (≥216 U/L) (LDH at disease progression) were associated with a significantly worse PFS. The Cox multivariate regression analyses indicated that a higher ELR (HR 0.399, 95% CI: 0.189–0.842, P=0.02) and a higher MLR (HR 2.162, 95% CI: 1.116–4.191, P=0.02) at the time of maximizing therapeutic benefits, a higher LDH (HR 2.456, 95% CI: 1.334–4.523, P=0.004) at the time of disease progression were independent risk factors for the PFS of advanced lung cancer patients treated with ICIs combined with platinum-based chemotherapy (Table S4, Figure 2).
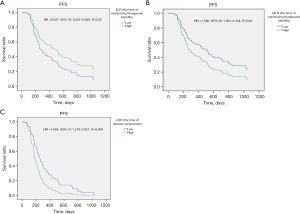
Discussion
The occurrence and progression of tumors are clarified to have a relationship with systemic inflammatory response. Immunotherapy changes the tumor microenvironment and stimulates immune cells to kill tumor cells by the activation of inflammatory response and regulation of immunological function, which is different from traditional therapies. Lymphocytes, the role of secreting cytokines and inducing cytotoxics (13), are essential immune cells in immunotherapy, especially tumor-specific T cells. Immunologic assessments in a phase I study showed that a redistribution of lymphocyte subsets from blood to tumor and tissue sites (14) occurred after nivolumab treatment. Not only lymphocytes but also neutrophils (15), monocytes, and eosinophils (16) may influence the outcome of immunotherapy through inflammatory response in several studies. Neutrophils, also regarded as influential effector cells of the inflammatory response, can release cytokines, chemokines, and growth factors to regulate the inflammatory microenvironment. That can promote the proliferation and migration of tumor cells (17). Monocytes belong to mononuclear phagocytes, which can transform into tumor-associated macrophages (TAM), including M1 and M2 subtypes, when mononuclear phagocytes trigger in a tumor microenvironment. The M2 subtype can promote the proliferation and migration of tumor cells and help tumor angiogenesis to promote tumor progression (18). Monocytes have been shown to be associated with the prognosis of colon cancer (19). Eosinophils directly affect tumor development and eliminate tumor cells by modulating the tumor microenvironment and promoting the infiltration of tumor-specific CD8-positive T cells (20). Platelet also takes part in the inflammation response through Toll-like receptors, they can be collected through binding with the tumor cell, then improve transforming growth factor beta (TGF-β) release, which can change tumor cells’ epithelial-mesenchymal transition to help tumor immune escape (21).
PD-L1 expression and TMB could mostly predict ICI efficacy, but not for all patients. Several inflammatory biomarkers, including NLR, MLR, ELR, and PLR, have been used in predicting efficacy (6-8). Some inflammatory predictive factors had different meanings at different therapy states in our study. It is well known that a high NLR before treatment is associated with a worse prognosis of immunotherapy (22). The clinical outcome of ICI combined with platinum-based chemotherapy did not correlate with NLR in our study. MLR at the time of maximizing therapeutic benefits (P=0.02) had a clear correlation with PFS. High MLR (≥0.3699) (HR 1.596, 95% CI: 1.091–2.334, P=0.02) was associated with a significantly worse PFS at the time of maximizing therapeutic benefits. It was an independent risk factor for the PFS.
Baseline eosinophils before treatment were independent potential biomarkers for PFS and overall survival (OS) in patients with NSCLC treated with nivolumab (23). It was proved in our study that there were significant correlations in ELR (P=0.02) before the second treatment and ELR (P=0.02) at the time of maximizing therapeutic benefits with PFS by Kaplan-Meier analysis. However, Cox proportional hazard regression analysis revealed that only high ELR (≥0.034) (HR 0.637, 95% CI: 0.437–0.929, P=0.02) at the time of maximizing therapeutic benefits which regarded as an independent risk factor was associated with a significantly better PFS.
When the treatment response reached maximizing therapeutic benefit, PLR (P=0.005) decreased significantly compared with before treatment. There was no association between clinical outcomes and PLR, that was not similar to the findings of the other study (24).
In addition, LDH serves as a crucial kinase in glycolysis pathways, which increases when the tumor grows rapidly in hypoxia. It means the glycolysis pathways improve to generate more energy when the tumor grows. So LDH elevation suggests a poor prognosis (25). Clinical data showed that the baseline LDH level was an independent risk factor for PFS in multivariate analysis (P=0.016) (26). There were significant correlations in LDH (P=0.003) at the time of disease progression with PFS by Kaplan-Meier analysis. Higher LDH (≥216 U/L) (HR 1.854, 95% CI: 1.218–2.821, P=0.004) at the time of disease progression regarded as another independent risk factor also tended to be associated with a worse PFS.
Most studies focused on the predictive values between baseline inflammatory biomarkers and therapy outcomes (7,27-30). Specifically, these results reflected the association between the baseline characteristics and the prognosis of therapy outcomes. In our study, the MLR and ELR at the time of maximizing therapeutic benefits and LDH at the time of disease progression had significant correlations with PFS of combination therapy. And there was no significant correlation between baseline inflammatory biomarkers with PFS. Our results mainly reflected dynamical prediction with therapy outcomes. Maybe the dynamical prediction biomarkers were more valuable than the baseline inflammatory biomarkers in predictive therapy outcomes.
Except for the meaningful results, we should pay attention to the limitations of the study. Firstly, the sample size was too small to draw definitive conclusions on the predictive value of inflammatory markers, and more data are necessary for further study. Secondly, our study belongs to retrospective analysis, and these meaningful results require to be confirmed in prospective observation. The advantage of the inflammatory markers needs to be clarified in the future.
Conclusions
This is the first study to exhibit the correlation between ICIs combined with platinum-based chemotherapy and inflammatory biomarkers in advanced lung cancers. The Cox multivariate regression analyses indicated that a higher ELR and a lower MLR at maximizing therapeutic benefits and a lower LDH at disease progression were positive protectors to the PFS of advanced lung cancer patients treated with the combination therapies. These meaningful results are simple, economical, easy to calculate, practical, and accessible from blood routine examinations. They are dynamic predictive detectors for advanced lung cancer patients receiving ICIs combined with platinum-based chemotherapy. They may help identify patients likely to benefit more from combination therapies.
Acknowledgments
The authors are grateful to the patients and their family and the investigators who participated in this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2198/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2198/prf
Funding: The study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2198/coif). S.L. received funding from foundation for the Outstanding Youth Training of 2021 in Tianjin Fourth Central Hospital (tjdszxyy20210007). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted according to the Declaration of Helsinki and its subsequent amendments and approved by the Ethics Committee of Tianjin Fourth Centre Hospital (No. SZXLL-2023-KY034). Tianjin Medical University Cancer Institute and Hospital was informed and agreed on the study. The informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol 2018;36:633-641. Erratum in: J Clin Oncol 2018;36:1645. [Crossref] [PubMed]
- Willis C, Fiander M, Tran D, et al. Tumor mutational burden in lung cancer: a systematic literature review. Oncotarget 2019;10:6604-22. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Valero C, Lee M, Hoen D, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun 2021;12:729. [Crossref] [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Winarno GNA, Mulyantari AI, Kurniadi A, et al. Predicting Chemotherapy Resistance in Gestational Trophoblastic Neoplasia: Ratio of Neutrophils, Lymphocytes, Monocytes, and Platelets. Med Sci Monit 2022;28:e938499. [Crossref] [PubMed]
- Graziano V, Grassadonia A, Iezzi L, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast 2019;44:33-8. [Crossref] [PubMed]
- Du S, Fang Z, Ye L, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts the benefit of gastric cancer patients with systemic therapy. Aging (Albany NY) 2021;13:17638-54. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573-7. [Crossref] [PubMed]
- Murthy P, Zenati MS, Al Abbas AI, et al. Prognostic Value of the Systemic Immune-Inflammation Index (SII) After Neoadjuvant Therapy for Patients with Resected Pancreatic Cancer. Ann Surg Oncol 2020;27:898-906. [Crossref] [PubMed]
- Fucà G, Guarini V, Antoniotti C, et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer 2020;123:403-9. [Crossref] [PubMed]
- Park W, Kwon D, Saravia D, et al. Developing a Predictive Model for Clinical Outcomes of Advanced Non-Small Cell Lung Cancer Patients Treated With Nivolumab. Clin Lung Cancer 2018;19:280-288.e4. [Crossref] [PubMed]
- Hirahara N, Matsubara T, Fujii Y, et al. Comparison of the prognostic value of immunoinflammation-based biomarkers in patients with gastric cancer. Oncotarget 2020;11:2625-35. [Crossref] [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Ferrucci PF, Gandini S, Battaglia A, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer 2015;112:1904-10. [Crossref] [PubMed]
- Delyon J, Mateus C, Lefeuvre D, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol 2013;24:1697-703. [Crossref] [PubMed]
- Şahin AB, Cubukcu E, Ocak B, et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep 2021;11:14662. [Crossref] [PubMed]
- Ma R, Ji T, Chen D, et al. Tumor cell-derived microparticles polarize M2 tumor-associated macrophages for tumor progression. Oncoimmunology 2016;5:e1118599. [Crossref] [PubMed]
- Li Z, Xu Z, Huang Y, et al. The predictive value and the correlation of peripheral absolute monocyte count, tumor-associated macrophage and microvessel density in patients with colon cancer. Medicine (Baltimore) 2018;97:e10759. [Crossref] [PubMed]
- Carretero R, Sektioglu IM, Garbi N, et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol 2015;16:609-17. [Crossref] [PubMed]
- Takemoto A, Okitaka M, Takagi S, et al. A critical role of platelet TGF-β release in podoplanin-mediated tumour invasion and metastasis. Sci Rep 2017;7:42186. [Crossref] [PubMed]
- Liu X, Hao N, Yang S, et al. Predictive factors and prognosis of immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer patients. Front Oncol 2023;13:1145143. [Crossref] [PubMed]
- Tanizaki J, Haratani K, Hayashi H, et al. Peripheral Blood Biomarkers Associated with Clinical Outcome in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J Thorac Oncol 2018;13:97-105. [Crossref] [PubMed]
- Ksienski D, Wai ES, Alex D, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for advanced non-small cell lung cancer patients with high PD-L1 tumor expression receiving pembrolizumab. Transl Lung Cancer Res 2021;10:355-67. [Crossref] [PubMed]
- Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010;29:625-34. [Crossref] [PubMed]
- Wei X, Zhang C, Zang F, et al. Preliminary study on inflammatory markers for predicting the efficacy and prognosis of anti-PD-1 antibody treatment in patients with non-small cell lung cancer. Chin J Clin Oncol 2021;48:547-52.
- Wang Z, Zhan P, Lv Y, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res 2019;8:214-26. [Crossref] [PubMed]
- Cortellini A, Ricciuti B, Borghaei H, et al. Differential prognostic effect of systemic inflammation in patients with non-small cell lung cancer treated with immunotherapy or chemotherapy: A post hoc analysis of the phase 3 OAK trial. Cancer 2022;128:3067-79. [Crossref] [PubMed]
- Wan M, Ding Y, Mao C, et al. Association of inflammatory markers with survival in patients with advanced gastric cancer treated with immune checkpoint inhibitors combined with chemotherapy as first line treatment. Front Oncol 2022;12:1029960. [Crossref] [PubMed]
- Muhammed A, Fulgenzi CAM, Dharmapuri S, et al. The Systemic Inflammatory Response Identifies Patients with Adverse Clinical Outcome from Immunotherapy in Hepatocellular Carcinoma. Cancers (Basel) 2021;14:186. [Crossref] [PubMed]


