Is internal mammary nodes irradiation as a part of breast cancer postoperative radiotherapy necessary?
Introduction
Breast cancer is the most common cancer among Chinese women for several years. It is also the main cause of cancer death for females (1,2). For breast cancer patients who had undergone breast-conserving surgery or a part of patients received modified radical mastectomy, radiotherapy plays a key role in the multidisciplinary treatment (3-5). However, it remains a controversy whether it is necessary to carry out internal mammary nodes (IMN) irradiation (IMNI) (6,7). In patients with breast cancer, the drainage areas of the IMN and the axillary lymph nodes have been regarded as the first nodal stations for lymphatic node drainage; however, the treatment for IMN drainage area remains controversial. Some randomized controlled trials (RCTs) have demonstrated that the extended resection of IMN did not improve the overall survival (OS) (8). Some earlier retrospective analyses also showed that the recurrence in IMN after surgery or systemic chemotherapy was rare and typically did not exceed 3% (9). For radiotherapy, the interests and controversies on IMNI are based on the findings of several recent clinical trials on radiotherapy for breast cancer (10-12); like some previous clinical trials, these trials emphasized the importance of controlling local breast cancer recurrence for long-term survival (13-16).
Clinical trials review
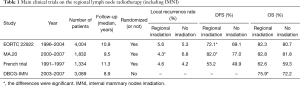
As shown in the 2005 EBCTCG meta-analysis, in patients who had received breast-conserving surgery, for every four recurrences avoided within 5 years by adjuvant radiotherapy, one breast cancer-specific death can be avoided 15 years after radiotherapy (i.e., 4:1 theory) (3). The 2014 EBCTCG meta-analysis further revealed the relationship between local control rate and long-term survival in patients with positive axillary lymph nodes after mastectomy (4). In addition, the EORTC 22922, a multicenter RCT, explored the value of local IMNI (11), For postoperative stages I, II, and III breast cancer patients in whom the tumors were located in the central area and the medial area, they were randomized into regional lymph nodes radiotherapy group (IMN plus supraclavicular lymph nodes) and non-regional lymph nodes radiotherapy group (control group). A total of 4,004 patients were enrolled. After 10 years of follow-up, the disease-free survival (DFS) in the regional lymph nodes radiotherapy group and control group was 72.1% and 69.1% (P=0.04), the distant metastasis-free survival (DMFS) was 78% and 75% (P=0.02), and the OS was 82.3% and 80.7% (P=0.06). This study indicated that the loco-regional lymph nodes radiotherapy, in particular IMNI, had a marginal positive effect on OS, and DFS was significantly improved.
The MA.20 study in Canada was another multicenter RCT that was similar to EORTC 22922 (12), although the primary endpoint of that study was to explore the effect of whole regional lymph nodes radiotherapy following breast-conserving surgery on the long-term survival rather than discussing the value of IMNI. A total of 1,832 patients who had undergone breast-conserving surgery from 2000 to 2007 were enrolled. The inclusion criteria were as follows: patients at high risk of relapse no matter the axillary lymph nodes were positive or negative. The risk factors included: the primary tumor was larger than 5 cm in size; the number of the dissected axillary lymph nodes were less than 10; in histological grade 3; ER-negative; and/or positive vascular tumor thrombus. All patients were randomized into breast radiotherapy following breast-conserving surgery group and breast plus regional lymph nodes radiotherapy group; in the latter group, the regional lymph nodes radiotherapy was defined as IMN plus axillary lymph nodes plus supraclavicular lymph nodes. After 10 years of follow-up, the DFS in the regional lymph nodes radiotherapy group and control group was 82.0% and 77.0% (P=0.01) and the OS was 82.8% and 81.8% (P=0.38). Although the MA.20 study showed that the regional radiotherapy (including IMNI) was beneficial for the DFS, the high local recurrence in no regional radiotherapy group was a notable problem in that study (Table 1). Among patients with recurrent disease, the lesions were located in the axillary lymph nodes in 63% of the patients and in the supraclavicular lymph nodes in 27% of the patients. Based on such prognostic data, we are doubted about the standards and quality of axillary lymph nodes dissection in the MA.20 study, because the recurrence rate in axillary lymph nodes following complete axillary lymph nodes dissection is often below 5% in general. There is also a possibility that the study included more patients with high risk, thus has a higher recurrence rate. Although the MA.20 study did not confirm that the loco-regional lymph nodes radiotherapy could benefit the OS in patients who had undergone breast-conserving surgery, the regional radiotherapy group did lower the loco-regional relapse (10-year locoregional DFS: IMLN/SCV LN RT 95.2% vs. no IMLN/SCV LN RT 92.2%; P=0.009). Further follow-up of the study may reveal whether there will be OS benefit after longer follow-up.
In contrast to the above two positive conclusions, Hennequin et al., a French team, carried out a multicenter randomized controlled trial on IMNI in 1991 and did not demonstrate that IMNI could benefit the patients in terms of local control or long-term survival (10). Stage I/II breast cancer patients with positive axillary lymph nodes or patients with central/medial tumors were enrolled in this study. All patients received postoperative irradiation of the chest wall and supraclavicular lymph nodes (irradiation of the apex of the axillary region in patients with positive axillary lymph nodes) and were randomly assigned to receive IMNI or not. The primary endpoint of the study was OS. After 10 years of follow-up, the DFS was 53.2% and 49.9%, respectively, in the IMNI group and control group, and the OS was 62.6% and 59.3%. Both DFS and OS showed no significant difference between these two groups. This trial failed to demonstrate a survival benefit for IMN irradiation. The findings were obviously contrary to the results of EORTC 22922 and MA.20. Notably, although the follow-up period was about 10 years in all these three studies, the OS was significantly lower in the French study than in the other two studies, which might because the French study was a relatively earlier study and the systemic treatment was somehow different from those in the other two studies.
A Danish group recently published the results of a large cohort study on IMNI (17); unlike the above three studies, the Danish research was not a randomized controlled trial; all the enrolled breast cancer patients were lymph nodes positive patients who had undergone breast-conserving surgery or modified radical mastectomy. According to the long adopted radiotherapy practice in this group, the breast and chest wall were irradiated, with the supraclavicular/subclavicular lymph nodes and axillary lymph nodes stations II/III. To avoid radiotherapy-associated cardiac toxicity, IMNI was performed in patients with right-sided disease, in whom the first to fourth intercostal spaces were irradiated by using electron-beam irradiation. Patients with left-sided disease were allocated to no IMNI. The primary endpoint was OS. A total of 3,377 patients were enrolled in this study. With a median of 8.9 years of follow-up time, the 8-year OS rates were 75.9% with IMNI versus 72.2% without IMNI. The effect of IMNI was more pronounced in patients at high risk of internal mammary nodes metastasis (the primary tumor was located in the central/medial area and/or the number of axillary lymph nodes was more than 4). Although the Danish research was not a prospective study, it met the “real-world” features (18). It revealed that IMNI could improve the OS (by 3.7% in 8 years); further analysis of the patients proportion showed that: there were more low-risk patients; 90% of the tumors were smaller than 5 cm in size; 60% of the patients had 1 to 3 positive ALNs; and 60% of the tumors were located in the lateral quadrant of the breast. On this basis, we can speculate that high-risk patients might benefit more from IMNI. As we know, targeted therapy with Herceptin is more feasible in breast cancer patients with HER2 overexpression (19); similarly, it is a new topic to screen high-risk patients or patients may benefit from IMNI in a simpler and faster way. Table 1 is a summary of the above four studies.
Full table
Based on the above three randomized controlled trials, it is difficult to evaluate the role of IMNI in the postoperative radiotherapy for breast cancer patients. Some recent observational studies presented evidences from the real world. Will the findings of these real world studies differed from the results of RCTs? In addition, with advances in technology and equipment, will IMNI bring new benefits to breast cancer patients? A recently published systematic review explored these questions (7). A total of four RCTs, four non-RCTs, seven retrospective studies, and 1 previously published meta-analysis were included in this systematic review. According to the authors, in some old RCTs, the differences in prognosis could not be identified in patients who had received IMNI or not due to limitations in technology or equipment (compared with the modern equipment) (7,10,20). Some newer RCTs, in contrast, have suggested that loco-regional lymph nodes radiotherapy (including IMNI) could improve the prognosis of patients by lowing the recurrence rate and even increasing the OS (7,11,12). Some non-RCTs and retrospective studies from the real word also indicated that radiotherapy (including IMNI) improved the prognosis (17,21,22). According to some recent studies, in the background of the wide use of modern radiotherapy technology and equipment, radiotherapy (including IMNI) increased the pulmonary complications; however, these adverse reactions were often mild. In addition, few data on cardiac toxicity have been available (7). The systematic review concluded that, based on the currently available evidences, the benefits and harms of postoperative radiotherapy should be balanced for each patient, and IMNI is recommended for high-risk patients whose tumor is located at the central/medial area or in patients with positive axillary lymph nodes.
Conclusions
In summary, the total risk of relapse should be considered during the decision-making on IMN radiotherapy; in particular, IMNI is recommended for high-risk patients whose tumor is located at the central/medial area or in patients with positive axillary lymph nodes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zeng H, Zheng R, Zhang S, et al. Female breast cancer statistics of 2010 in China: estimates based on data from 145 population-based cancer registries. J Thorac Dis 2014;6:466-70. [PubMed]
- Levaggi A, Poggio F, Lambertini M. The burden of breast cancer from China to Italy. J Thorac Dis 2014;6:591-4. [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]
- Xu B, Hu X, Jiang Z, et al. National consensus in China on diagnosis and treatment of patients with advanced breast cancer. Transl Cancer Res 2015;4:557-73. [PubMed]
- Vermeulen SS, Haas JA. CyberKnife stereotactic body radiotherapy and CyberKnife accelerated partial breast irradiation for the treatment of early breast cancer. Transl Cancer Res 2014;3:295-302.
- Verma V, Vicini F, Tendulkar RD, et al. Role of Internal Mammary Node Radiation as a Part of Modern Breast Cancer Radiation Therapy: A Systematic Review. Int J Radiat Oncol Biol Phys 2016;95:617-31. [Crossref] [PubMed]
- Veronesi U, Marubini E, Mariani L, et al. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. Eur J Cancer 1999;35:1320-5. [Crossref] [PubMed]
- Chen RC, Lin NU, Golshan M, et al. Internal mammary nodes in breast cancer: diagnosis and implications for patient management -- a systematic review. J Clin Oncol 2008;26:4981-9. [Crossref] [PubMed]
- Hennequin C, Bossard N, Servagi-Vernat S, et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys 2013;86:860-6. [Crossref] [PubMed]
- Poortmans PM, Collette S, Kirkove C, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 2015;373:317-27. [Crossref] [PubMed]
- Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:307-16. [Crossref] [PubMed]
- Zhou ZR, Mei X, Chen XX, et al. Systematic review and meta-analysis comparing hypofractionated with conventional fraction radiotherapy in treatment of early breast cancer. Surg Oncol 2015;24:200-11. [Crossref] [PubMed]
- Zhang L, Yang ZZ, Chen XX, et al. Dose coverage of axillary level I-III areas during whole breast irradiation with simplified intensity modulated radiation therapy in early stage breast cancer patients. Oncotarget 2015;6:18183-91. [Crossref] [PubMed]
- Wöckel A, Wolters R, Wiegel T, et al. The impact of adjuvant radiotherapy on the survival of primary breast cancer patients: a retrospective multicenter cohort study of 8935 subjects. Ann Oncol 2014;25:628-32. [Crossref] [PubMed]
- Donker M, Litière S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol 2013;31:4054-9. [Crossref] [PubMed]
- Thorsen LB, Offersen BV, Danø H, et al. DBCG-IMN: A Population-Based Cohort Study on the Effect of Internal Mammary Node Irradiation in Early Node-Positive Breast Cancer. J Clin Oncol 2016;34:314-20. [Crossref] [PubMed]
- Revicki DA, Frank L. Pharmacoeconomic evaluation in the real world. Effectiveness versus efficacy studies. Pharmacoeconomics 1999;15:423-34. [Crossref] [PubMed]
- Montemurro F. Should trastuzumab be administered concomitantly with anthracycline in women with early, HER2-positive breast cancer? Transl Cancer Res 2014;3:541-6.
- Kaija H, Maunu P. Tangential breast irradiation with or without internal mammary chain irradiation: results of a randomized trial. Radiother Oncol 1995;36:172-6. [Crossref] [PubMed]
- Veronesi U, Arnone P, Veronesi P, et al. The value of radiotherapy on metastatic internal mammary nodes in breast cancer. Results on a large series. Ann Oncol 2008;19:1553-60. [Crossref] [PubMed]
- Stemmer SM, Rizel S, Hardan I, et al. The role of irradiation of the internal mammary lymph nodes in high-risk stage II to IIIA breast cancer patients after high-dose chemotherapy: a prospective sequential nonrandomized study. J Clin Oncol 2003;21:2713-8. [Crossref] [PubMed]


