Did dose escalated radiotherapy in stage III non-small cell lung cancer improve overall survival?
Introduction
Lung cancer has been a leading cause for both male and female cancer patients worldwide (1,2). Unfortunately many of them would be diagnosed as advanced stage patients without the chance to be treated by surgery. Radiotherapy can be their alternative choice (3,4). In addition, radiotherapy has an important role in the palliation of metastatic non-small cell lung cancer (NSCLC) (5). In the 1980s, Perez launched a randomized controlled study (RTOG 7301) of various irradiation doses and fractionation schedules in the treatment of inoperable non-small-cell lung cancer. In this study, 60 Gy was firstly consolidated as an optimal dosage for radiotherapy which yielded higher tumor response, better survival and less intra thoracic recurrence than other less than 60 Gy groups (6). Since then, 60 Gy radiotherapy has been commonly accepted in treatment of advanced stage NSCLC patients. In the following clinical trial (7), concurrent radiochemotherapy has been confirmed to be beneficial for NSCLC patients. Some researchers believe that increased dosage may lead to better local control rate and longer overall survival (OS). However both acute and late toxicity results from high dosage may occur, which may hinder treatment plan and be life threatening sometimes. Therefore, several studies have been launched to explore its efficacy and its safety in clinical practice.
Clinical trials review
In 2004, Socinski (8) had performed a dose escalation phase I study with the help of three-dimensional thoracic conformal radiation therapy (TCRT). In this study, all entered 29 patients were suffering from stage III non-small-cell lung cancer and 25 patients were considered assessable for evaluating the tolerability and toxicity. The TCRT dosage was escalated from 78 to 90 Gy without dose-limiting toxicity. The average maximum dose to the esophagus was 77.7 Gy (range, 70.9 to 90.0 Gy). Grade 3 esophagitis happened in four patients and this escalation of the TCRT was accomplished safely in this study. This study has confirmed that high dosage radiotherapy can be tolerated in inoperable stage III non-small-cell lung cancer patients. In 2005, Bradley (9) published the results of their three-dimensional radiotherapy dose-escalation phase I–II study (RTOG 9311). One hundred and seventy-nine patients entered this study and 177 patients were eligible. Among them, 83 patients were stage III NSCLC patients. In this cohort, patients were stratified at escalating radiation dose levels depending on the percentage of the total lung volume that received >20 Gy with the treatment plan (V20). Patients with a V20 <25% (group 1) received 70.9, 77.4, 83.8, and 90.3 Gy successively. Patients with a V20 of 25–36% (group 2) received doses of 70.9 and 77.4 Gy, successively. The following acute grade 3 or worse toxicities were observed for group 1: 70.9 Gy (1 case of weight loss), 77.4 Gy (nausea and hematologic toxicity in 1 case each), 83.8 Gy (1 case of hematologic toxicity), and 90.3 Gy (3 cases of lung toxicity). The following acute grade 3 or worse toxicities were observed for group 2: none at 70.9 Gy and 2 cases of lung toxicity at 77.4 Gy. No patients developed acute grade 3 or worse esophageal toxicity in group 2. In this study, the radiation dose was safely escalated to 83.8 Gy using three-dimensional conformal techniques. The 90.3-Gy dose level was too toxic, resulting in dose-related deaths in two patients. Its safety has also been consolidated in other studies (10,11). Also, locoregional control was achieved in 50–78% of patients in this study.
Since high dosage radiotherapy is safe in patients and could be beneficial because of relatively high locoregional control, several studies were launched to explore whether high dosage radiotherapy could improve lung cancer patients survival. In 2005, Kong et al. (12) published results from their phase I clinical investigation, which suggested high dosage radiotherapy would improve the OS of lung cancer patients. One hundred and six patients were enrolled in this study and 57% patients in this cohort were stage III patients. All these patients were assigned in three groups: group 1 with 63–69 Gy; group 2 with 74–84 Gy; and group 3 with 92–103 Gy. Median survival time (MS) and time to locoregional progression were important endpoints in this analysis. MS for these three groups were 11.7 months (group 1), 26.7 months (group 2) and 22 months (group 3) respectively, with significantly differences (P=0.0002). Locoregional progression-free survival were 10 months (group 1), 23.3 months (group 2) and 22 months (group 3) respectively (P=0.0228). In this study, group 2 showed quite promising effect. This investigation demonstrated that radiation dose was an independent predictor for both OS and locoregional tumor control. With a dose increase of 1 Gy, the risk from death was reduced by a factor of 3%. However, the side effects extremely due to high dosage would negatively affect survival of patients. Curran (13) had launched a phase III trial (RTOG 9410). In this study, 610 patients were randomly assigned to two concurrent regimens and one sequential chemotherapy and TCRT regimen [arm 1: 60 Gy + concurrent chemotherapy; arm 2: 60 Gy + sequential chemotherapy; arm 3: 69.6 Gy (1.2 Gy twice-daily) + concurrent chemotherapy]. Five hundred and sixty-six patients in this study were diagnosed as stage III lung cancer patients. MS time were 14.6, 17.0, and 15.6 months for arms 1–3, respectively (arm 1 vs. arm 2: P=0.046; arm 1 vs. arm 3: P=0.46). This prospective phase III study consolidated the efficacy of concurrent chemotherapy in the treatment of advanced stage lung cancer patients. However, hyper fractionated radiation with relative high dosage did not benefit patients. Both these two clinical trials were involved with high dosage radiotherapy, but these outcomes were contradictory.
More studies were performed to investigate whether high dosage radiotherapy would benefit lung cancer patients. In 2015, Bradley published a report from a prospective phase III trial (RTOG 0617) (14). There were 544 stage III lung cancer patients enrolled. All these patients were randomly assigned in four groups: 60 Gy (standard dose), 74 Gy (high dose), 60 Gy plus cetuximab and 74 Gy plus cetuximab. Radiation dose was prescribed to the planning target volume and was given in 2 Gy daily fractions with either intensity-modulated radiation therapy or three-dimensional conformal radiation therapy. Median OS was 28.7 months (95% CI: 24.1–36.9) for patients who received standard-dose radiotherapy and 20.3 months (95 CI: 17.7–25.0) for those who received high-dose radiotherapy [hazard ratio (HR) 1.38; 95% CI: 1.09–1.76; P=0.004]. However, severe esophagitis was more common in patients who received high-dose radiotherapy than in those who received standard-dose treatment [43 (21%) of 207 patients vs. 16 (7%) of 217 patients; P<0.0001]. High dosage radiotherapy would not improve the outcome of these patients but accompanied with sever radiation resulted side effects. This was quite incompatible with the previous results from phase I or II which led to this clinical trial, but it was quite similar to that of trial RTOG 9410.
Brower (15) did a retrospective study on this topic. A total of 33,566 patients with stage III NSCLC treated with chemoradiation from 2004–2012 and radiation doses between 59.4–85 Gy were included. Patients were stratified by dose with median OS of: 18.8, 19.8 and 21.6 months for cohorts receiving 59.4–60, 61–69, and ≥70 Gy respectively (P<0.001). While 66, 70 and ≥71 Gy resulted in increased OS in comparison to 59.4–60 Gy, no significant difference in OS was observed when comparing 66 to ≥71 Gy (P=0.38). In this study, the results showed that increased dosage would improve OS in stage III NSCLC. However, this was opposite opinion to that of trial RTOG 0617. Table 1 summarizes the baseline characteristics of the main clinical studies on this topic.
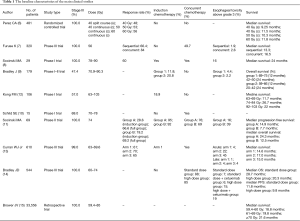
Full table
Discussion
From the results of clinical trials above, we would be very confused on these. In general, higher dosage radiotherapy is believed to be more lethal for cancer cells, which may contribute to better locoregional control and longer survival time. However its toxicity for lungs, esophagus and trachea should not be ignored. RTOG 0617 was a phase III prospective study, which was considered to be a relatively high quality evidence. Even so, when RTOG 0617 results were used to guide clinical practice, these should be interpreted carefully for reasons as follow. First, patients from this cohort were treated with only two kinds of radiotherapy dosage. Standard and high dosages were regimens with a relatively big interval, which means some possible beneficial dosage above 60 Gy may be skipped. As a newly published prospective study, relatively new machines and techniques may kill cancer cells in the filed more precisely and sharply. Also, advanced technologies mean normal tissues (heart, lung and esophagus) exposed in the filed could be harmed significantly when compared with the same dosage applied in former clinical trial. The high dosage radiotherapy originated toxicity may negatively affect its positive role in eliminating cancer cells, which appears as relatively shorten OS. This hypothesis has been consolidated for the occurrence rate of esophageal toxicity above grade 3 in RTOG 0617 was obviously higher than that of Bradley’s clinical investigation (9) (2.2–2.4% vs. 15–19%). Also, in RTOG 0617 multivariate models generated with heart V5 (the percentage of heart volume receiving ≥5 Gy) and V30, on separate multivariate analysis were both important predictors of patient survival.
Brower’s study was with the greatest number among all these studies though it’s retrospective one. Except for biases, some important markers such as tumor or nodal volume, locoregional control rate and toxicity were absent, which can be a confounding factor in analysis and may affect its results. Relative long time span in this retrospective study also means different concurrent chemotherapy and various chemotherapy regimens applied as induction and sequential treatment, which could also be a confounding factor for analysis.
In addition, in our review, we only care about the total radiation dose, and did not pay special attention to dose fractionation, in fact, according to previous studies, different dose fractionation doesn’t seem to have much impact on patients’ prognosis (16,17). In summary, increased dosage radiotherapy might benefit stage III NSCLC patients. However its toxicity should not be ignored. Therefore, standard dosage radiotherapy is strongly recommended in clinical practice. With better protection tactics, high-dosage radiotherapy could be applied for special patients, such as with high risk. In addition, we also think relatively new toxicity-tolerated prospective study and potential risk factor screening investigation are urgently needed in order to find out candidates who may benefit from high dosage radiotherapy.
Conclusions
Standard dose radiotherapy (60 Gy) is classical protocol in the treatment of stage III NSCLC patients. Before solid evidence show up, high dosage radiotherapy should be only recommended in special patients, and good protection tactics are needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Bernard ME, Clump DA, LaLonde R, et al. Radiation therapy for locally advanced lung cancer. Transl Cancer Res 2015;4:356-71.
- Lazarus MA, Schachter L, Xavier M. Social factors, treatment, and survival in patients with advanced-stage non-small cell lung cancer. Transl Cancer Res 2014;3:146-51.
- Kapoor R, Simha V. Advances in palliative radiotherapy of metastatic non-small cell lung cancer. Transl Cancer Res 2015;4:397-402.
- Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer 1980;45:2744-53. [Crossref] [PubMed]
- Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692-9. [PubMed]
- Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol 2004;22:4341-50. [Crossref] [PubMed]
- Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2005;61:318-28. [Crossref] [PubMed]
- Schild SE, McGinnis WL, Graham D, et al. Results of a Phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:1106-11. [Crossref] [PubMed]
- Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 2008;26:2457-63. [Crossref] [PubMed]
- Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:324-33. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Brower JV, Amini A, Chen S, et al. Improved survival with dose-escalated radiotherapy in stage III non-small-cell lung cancer: analysis of the National Cancer Database. Ann Oncol 2016;27:1887-94. [Crossref] [PubMed]
- Haslett K, Pöttgen C, Stuschke M, et al. Hyperfractionated and accelerated radiotherapy in non-small cell lung cancer. J Thorac Dis 2014;6:328-35. [PubMed]
- Zhang W, Liu Q, Dong X, et al. A meta-analysis comparing hyperfractionated vs. conventional fractionated radiotherapy in non-small cell lung cancer. J Thorac Dis 2015;7:478-85. [PubMed]


