Nanoparticle-mediated arresten gene inhibits neointimal formation of vein grafts: an experimental research
Introduction
Autologous vein graft is the main vessel passage of peripheral ischemia in patients for treating chronic obstructive disease of the lower limbs of long or multiple segmental small blood vessels, but the disease is unable for interventional therapy (1,2). Studies on this area abroad have shown that vein graft restenosis is the main and leading cause of death and disability. In the future, restenosis and even occlusion would develop in the lumen of the vein-bridge in 20–50% of vein grafts; and surgery would eventually lead to failure (3,4). These changes would bring difficulties to clinical treatment. In this experiment, rat experimental models of autogenous vein grafts were established to observe pathological changes in the vascular tissues and new intima hyperplasia of experimental vascular segments, and to study the expression of MMP-2 in vascular intima and the hyperplasia of the intima.
Methods
Experimental materials
Animal
Thirty adult female Wistar rats, weighting 250±10 g, were purchased from the Animal Experiment Center of Shandong University (Experimental animal certificate).
Laboratory reagents
H&E staining fluid were bought from Solarbio, SP Mouse HRP Kit purchased from CWBIO, Citrate Buffer (100×) was bought from CWbio, MMP-2 polyclonal antibody was purchased from Proteintech, and the arresten gene was compounded by HANBIO. The nanoparticle-mediated arresten gene was compounded by Taishan Medical College of Pharmacy, and 10% chloral was bought from Qilu Hospital of Shandong University. Heparin sodium and Gentamycin sulfate injections were bought from Jinan Central Hospital.
Experimental apparatus
Microscope, microscopic tweezers and microscopic needle clamps were all bought from Ningbo Medical Suture Needle Co., Ltd. The 22 G intravenous catheter needles were provided by Jinan Central Hospital. The operating microscope was bought from Jiangsu Zhenjiang Zhongtian Optical Instrument Co., Ltd.
Experimental test method
Animal groups
Thirty healthy female Wistar rats were randomly divided into three groups by random number table method: group A, nanoparticle-mediated arresten gene; group B, empty nanoparticles group; group C, the blank group (Table 1).
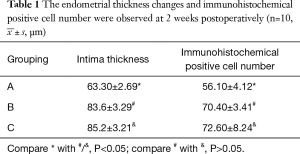
 µm)
µm) Full table
Preparation of the model
All animal tests were performed according to the agreements approved by the Institutional Committee for the Use and Care of Laboratory Animals. Mice were bred in a light/dark (12-hour/12-hour) cycle at 20–22 °C with a humidity of 45–55% and fed with food and water arbitrarily. All rats were fed for seven days to adapt with the environment. Rats were weighed before the surgery. The center of the neck was disinfected, shaved and prepped in a sterile fashion, usually with a bated iodine solution, under 4% chloral hydrate anesthesia. The skin and underlying tissues were incised, and the external jugular veins and branches were separated. Surrounding tissues were separated through an operating microscope and micro forceps. Venous blood flow was blocked with noninvasive vascular clamps, and the distal of the external jugular vein were truncated. Heparin water was used to flush the venous lumen. The sheath pipe of a 22 G intravenous catheter needle was used to prepare the vascular cuffing (length of approximately 6 mm). At the end of the cuffing, approximately 2 mm was grooved for ligaturing the blood vessel. In anesthetic conditions, right-sidedness common carotid arteries were separated, which was to be snipped. A traction line of 11-0 was seamed in the broken end of common carotid artery. The cuffing was inserted into the broken ends of the vessels by a traction line of 11-0. With the assistance of no tooth tweezers, the artery was flipped outward to the cuffing. The vessels were fixed, and the telecentric end of the inverted vein was inserted into the artery. Arteries and veins were be ligated in the groove by a nylon line of 11-0. The proximal part of the vein was cut out and washed by heparin water. The proximal part of external jugular vein was connected to the common carotid artery in the same way, and was tied with a nylon line. The transplanted vein was identified to be a strong ligation. The noninvasive vascular vein was slowly loosened to restore blood flow to the vascular bridge. The color of the vein bridge was red, which was obviously filled and pulsatile (Figure 1). These results indicate that the blood vessels are unblocked, suggesting that the model of vein grafts was successfully established. The incision was sewed up after the incision without oozing blood. Gentamicin was abdominally injected after the operation to prevent infection, and was applied for three consecutive days. After the operation, the following were applied for two weeks: group A, subcutaneous injection of nanoparticle-mediated arresten gene (0.2 mL); group B, subcutaneous injection of blank nanoparticles (0.2 mL); group C, subcutaneous injection of saline (0.2 mL). Growth conditions of rats were observed daily.
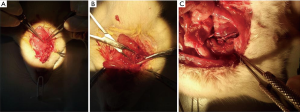
Specimen collection
After two weeks, rats were anesthetized with 4% chloral hydrate again, the skin was incised according to the original incision, the vein graft was separated, and 1 cm of the vein was taken and divided into two. A portion of the tissues were immediately fixed in 4% paraformaldehyde for 12–24 h, paraffin-embedded after dehydration, and serially cut for histologic examination. A part of the vain was immediately placed in a refrigerator at −80 °C.
Main outcome measures
(I) Morphological observation of pathological tissue serial sections was stained by H&E. The thickness of the lining were measured by a computer image analysis system; (II) immunohistochemistry was used to examine the expression of MMP-2 in tissue samples of the vein graft.
Statistical analysis
Experimental data were analyzed by SPSS 20.0 software and expressed as mean ± standard deviation ( ). Analysis of variance (ANOVA) was used to analyze the data of these groups. A P value <0.05 was considered statistically significant.
). Analysis of variance (ANOVA) was used to analyze the data of these groups. A P value <0.05 was considered statistically significant.
Results
Morphological observation of pathological tissues
Intimal hyperplasia was found in different degrees in all specimens of autogenous vein grafts. Varying degrees of narrowing could occur. The lining vein obviously thickened, and the degree of thickening was uneven (H&E staining results, Figure 2). Neointimal hyperplasia in groups B and C were evident, and was slightly observed in group A. The intima of groups B and C were more obviously hyperplastic than in group A (P<0.05), and there was no significant difference between groups B and C (P>0.05).
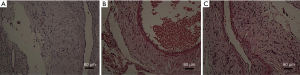
Immunohistochemical staining results
Immunohistochemistry was applied to detect the expression of MMP-2 in endothelial cells (results are shown in Figure 3).

Discussion
Vascular restenosis is a repair response after vascular injury. The original vein was exposed in arterial circulation, high artery blood flow and high pressure conditions; and a complex series of pathophysiological changes occurred. However, the mechanism of restenosis remains unclear, which depends on the alteration of multi-factors, multi-stages and multi-genes. Part of it is an adaptive response such as vascular dilation, thickening of the wall and venous arterialization, while the other part is an overreaction such as intimal hyperplasia (5); eventually leading to intravascular thrombosis and even occlusion (6). Among the most significant was that blood vessel smooth muscle cells (SMCs) has the phenotype shift by means of cell factors modulation. SMCs migrated into the injured site and proliferated, leading to intimal hyperplasia and vessel wall remodeling, which induce restenosis. According to the research of a foreign scholar in the process of their transplanted vein remodeling, the degradation of the extracellular matrix (ECM) is a necessary process, in which SMCs migrate into the new lining layer. Matrix metalloproteinases (MMP) is the key enzyme of ECM degradation. Thus, determining how to reduce the expression of MMP is the key to the inhibition of neointimal hyperplasia. After the success of the experiment in vascular transplantation, rats in the experimental group were subcutaneously injected with nanoparticles to mediate the arresten gene; then, pathological changes of local vascular tissues, the new intima hyperplasia of experimental vascular segments, and MMPS expression were observed. The study obtained the following results: arresten inhibited excessive thickening of the vascular intima effectively, which may partly be related to its inhibition on the secretion of MMP-2 to inhibit the proliferation of VSMCs; thus, reducing intimal and medial hyperplasia in the graft.
Arresten was found by Colorado in 2000, which is an endogenous inhibitor of angiogenesis derived from collagen IV. It has the gene code for a 16.5 KD protein and consists of approximately 272 amino acid residues. It is the polypeptide fragments of the domain of carboxyl terminal NC1, which is the α1 chain of type IV collagen (7,8). The arresten gene plays a crucial role in the accumulation of type IV collagen, the formation of basement membranes, and regulation of cell biology (9,10). It inhibits endothelial cell and vascular SMC proliferation and migration, and induces the apoptosis of endothelial cells. Many studies have found that media SMCs may be the origin of cells of transplanted neointimal veins. As a result, these cells exist in the ECM framework and only degrade ECM (11) surrounding cells. Thus, cells in the media and adventitia can migrate; and in the end, the vessels can be restored. MMP are key enzymes in the degradation of the ECM (12) and the remodeling of the vascular wall. They are zinc dependent proteolytic enzymes that have a specific role in degrading the ECM (13,14). Thus, reducing the expression of these enzymes is a means of preventing excessive neointimal hyperplasia. MMPs are a family of enzymes that proteolytically degrade various components of the ECM such as type IV collagenases. MMP-2 can degrade components such as type IV collagen, fibronectin, elastin and denatured interstitial collagen (15). Mason found that MMP-2 can promote the migration of SMC, inhibit the invasion and migration of human retinal microvascular endothelial cells, and change vascular remodeling (16,17). MMP-2, which is localized in cardiomyocytes, can degrade type-IV collagen and other bioactive molecules, and is overexpressed in head and neck squamous cell carcinomas during cancer invasion and metastasis (18). A survey of available literature shows that MMP-2 expression was suppressed with RNA interference (RNAi), and its inhibitory effects were observed on the invasion and migration of human retinal microvascular endothelial cells (19). MMP-2 upregulation has been implicated in the pathophysiology of atherosclerosis (20). H&E staining and immunohistochemical staining revealed that the process of the endometrial hyperplasia arresten gene could inhibit the production of MMP-2, to further prove that the process of neointimal hyperplasia may be related to the degradation of the ECM.
In particular, this experiment explored a new research field of vein graft and established an experimental animal model of external carotid vein transplant to the carotid artery. This is a practical model with a high achievement ratio and rare complications, which can be used in further studies on vein graft. In addition, the experiment confirmed that the growth and migration of vascular smooth-muscle cells resulted in neointimal proliferation after vascular transplantation, which is the key mechanism of restenosis after vascular grafts. The degradation of the ECM is a necessary process, in which SMCs migrate into the new lining layer. The arresten gene can inhibit this process to some degree.
Acknowledgements
Funding: The National Natural Science Foundation of Shandong (Grants ZR2009CM052) Science and Technology Boart of Jinan City (Grants 201401079) and The Science and Technology Project of Jinan City Health Bureau (Grants 2007-32 and 2008-11) are gratefully acknowledged for the financial support to this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Affiliated Jinan Central Hospital of Shandong University (No. ZR2009Cm052).
References
- King P, Royle JP. Autogenous vein grafting in atheromatous rabbits. Cardiovasc Res 1972;6:627-33. [Crossref] [PubMed]
- Wyatt AP, Rothnie NG, Taylor GW. The vascularization of vein-grafts. Br J Surg 1964;51:378-81. [Crossref] [PubMed]
- Veith FJ, Gupta SK, Ascer E, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg 1986;3:104-14. [Crossref] [PubMed]
- Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. [Crossref] [PubMed]
- Kim YO, Choi YJ, Kim JI, et al. The impact of intima-media thickness of radial artery on early failure of radiocephalic arteriovenous fistula in hemodialysis patients. J Korean Med Sci 2006;21:284-9. [Crossref] [PubMed]
- Engelse MA, Lardenoye JH, Neele JM, et al. Adenoviral activin a expression prevents intimal hyperplasia in human and murine blood vessels by maintaining the contractile smooth muscle cell phenotype. Circ Res 2002;90:1128-34. [Crossref] [PubMed]
- Sudhakar A, Nyberg P, Keshamouni VG, et al. Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J Clin Invest 2005;115:2801-10. [Crossref] [PubMed]
- Boosani CS, Nalabothula N, Sheibani N, et al. Inhibitory effects of arresten on bFGF-induced proliferation, migration, and matrix metalloproteinase-2 activation in mouse retinal endothelial cells. Curr Eye Res 2010;35:45-55. [Crossref] [PubMed]
- Zhang WD, Bai HZ, Sawa Y, et al. Association of smooth muscle cell phenotypic modulation with extracellular matrix alterations during neointima formation in rabbit vein grafts. J Vasc Surg 1999;30:169-83. [Crossref] [PubMed]
- Walsh K, Smith RC, Kim HS. Vascular cell apoptosis in remodeling, restenosis, and plaque rupture. Circ Res 2000;87:184-8. [Crossref] [PubMed]
- Mecham RP, Broekelmann TJ, Fliszar CJ, et al. Elastin degradation by matrix metalloproteinases. Cleavage site specificity and mechanisms of elastolysis. J Biol Chem 1997;272:18071-6. [Crossref] [PubMed]
- Strilakou A, Perelas A, Lazaris A, et al. Immunohistochemical determination of the extracellular matrix modulation in a rat model of choline-deprived myocardium: the effects of carnitine. Fundam Clin Pharmacol 2016;30:47-57. [Crossref] [PubMed]
- Apte SS, Parks WC. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol 2015;44-46:1-6. [Crossref] [PubMed]
- Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 2010;20:161-8. [Crossref] [PubMed]
- Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: A. C. Lockhart et al., Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res., 9: 00-00, 2003. Clin Cancer Res 2003;9:551-4. [PubMed]
- Mason DP, Kenagy RD, Hasenstab D, et al. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ Res 1999;85:1179-85. [Crossref] [PubMed]
- Gu Y, Ke G, Wang L, et al. Silencing Matrix Metalloproteinases 9 and 2 Inhibits Human Retinal Microvascular Endothelial Cell Invasion and Migration. Ophthalmic Res 2015;55:70-5. [Crossref] [PubMed]
- Hughes BG, Schulz R. Targeting MMP-2 to treat ischemic heart injury. Basic Res Cardiol 2014;109:424. [Crossref] [PubMed]
- Gu Y, Ke G, Wang L, et al. Silencing Matrix Metalloproteinases 9 and 2 Inhibits Human Retinal Microvascular Endothelial Cell Invasion and Migration. Ophthalmic Res 2015;55:70-5. [Crossref] [PubMed]
- Azevedo A, Prado AF, Antonio RC, et al. Matrix metalloproteinases are involved in cardiovascular diseases. Basic Clin Pharmacol Toxicol 2014;115:301-14. [Crossref] [PubMed]


