The role bronchoscopy in the diagnosis of airway disease in children
Introduction
Bronchoscopy allows direct visualization of trachea and bronchi by rigid open tube bronchoscope or flexible fiberoptic scope (1). Detailed evaluation of airways with bronchoscopy offers advantages over other diagnostic tools and allows interventional procedures such as biopsy of lesions, removal of foreign bodies, dilatations of stenosis and obtaining samples for cytological and microbiologic analysis. Bronchoscopy can be performed either by rigid (RB) or flexible (FB) instruments depending on the needs of patients. The pediatric airway is notably different from adults. It is smaller in size, larynx and tracheal proportion is more as compared to adults and epiglottis is more posterior and narrower (2). Therefore, type of bronchoscopy should be decided not only for indications but also considering the procedure specific instrumentations.
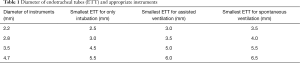
FB enables to obtain anatomical and dynamic information of airways and offers sampling from distal airways for cytological and microbiological studies (3). It is carried out under light sedation or general anesthesia. During the procedure, patient can be spontaneously breath around a small FB and positive pressure ventilation can be needed under some circumstances. Ventilation can be also assisted via laryngeal mask, nasopharyngeal or endotracheal tube (4). Diameters of FB and endotracheal tubes are listed in Table 1 (5). Pediatric FB are 1.8 to 4.9 mm in size with suction channels. Biopsy forceps and cytology brushes are also available for 3.5 mm and larger bronchoscopes (6). A 3.5 mm FB can be used for neonates, children and adults. It has also suction ports for bronchoalveolar lavage (BAL) but they have limited role in foreign body removal. Only small superficial tissues can be sampled with 3.5 mm bronchoscopes. A 4.7 mm FB can be used in children older than 6 years of age.
Full table
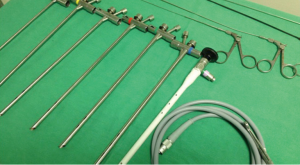
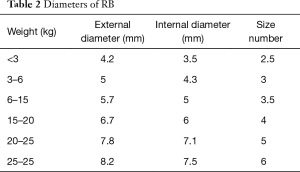
RB are performed under general anesthesia in an operating theatre setting. In children, RB can be used not only for diagnosis but also for therapeutic purposes. The main advantage of RB is that it secures the airway and allows for assisted ventilation during the procedure. RB are 3 to 7 mm in diameter and 20–50 cm in length (Figure 1). External diameter of RB is selected according to the weight of the child (Table 2) (7). Rigid telescopes such as direct 0° and angled (30°–70°) are available with 2.7 and 4 mm diameters.
Full table
After a brief introduction of FB and RB, it is aimed to review the role of bronchoscopy in the diagnosis of airway disease in children.
Indications of bronchoscopy
Bronchoscopy is utilized to define airway anatomy and airway dynamics, to obtain specimens for further diagnostic study. Indications of bronchoscopy are exploration of airways, obtaining biological samples and therapeutic applications (Table 3) (3).

Full table
Evaluating the airway anatomy and dynamics
There are several causes indicating exploration of the airways. Laryngomalacia is main cause of persistent stridor and requires FB in case of atypical presentation, biphasic character, history of difficult intubation and suffocation crisis (8). Hemoptysis is uncommon in children and mostly associated with artificial airways. Unexplained hemoptysis requires FB to rule out endobronchial pathologies. Persistence of atelectasis more than 6 weeks with unexplained symptoms makes FB recommendable (9). If localized hyperlucency in chest X-ray is not associated with congenital or infectious causes, air trapping due to an intrinsic obstruction or extrinsic compression should be evaluated with FB. During FB, deep sedation should be avoided if air dynamics are evaluated for laryngomalacia, tracheomalacia and bronchomalacia. It is also possible to evaluate vocal cord granulomas, endotracheal or tracheostomy tube complications and laryngeal cysts (10).
Obtaining biological samples
BAL is the most common method to obtain specimen from distal airways and alveolar surfaces. It is the most important aspect of diagnostic bronchoscopy. Saline is installed into distal airways and fluid returned from the lavage is collected to measure the soluble and cellular contents alveolar surface. Since epithelial fluid is not static, it is difficult to estimate the true concentration of substances. It depends on duration and volume of the fluid employed during lavage. Therefore, specimens obtained by BAL are more useful to evaluate infectious and inflammatory process than quantitative analysis. The indications of BAL are (5):
- Diagnosis of suspected infection;
- Pulmonary infiltrates;
- Dyspnea;
- Hypoxia;
- Tachypnea;
- Recurrent and/or persistent pulmonary infiltrates;
- Interstitial infiltrates;
- Diffuse alveolar infiltration;
- Pulmonary hemorrhage;
- Alveolar proteinosis;
- Suspected aspiration;
- Lung transplant;
- Hypereosinophilic lung disease.
The other indication of BAL is to obtain fluid for diagnosis of infection processes when the sputum cannot be obtained in children. Also immunocompetent patients and children with cystic fibrosis may be unable to produce sputum for microbiological analysis (11). BAL can be obtained in these children to investigate atypical mycobacteria and more reliable than sputum cultures. BAL ideally should be obtained before starting antimicrobial therapy but can be still informative if the patient is unresponsive to treatment or deteriorating in spite of antimicrobial treatment.
The other important indication of BAL is to define the aspiration. Presence of significant number of macrophages heavily laden with lipid may support the diagnosis of aspiration (12). Pepsin in BAL samples is also indicative for aspiration. In lung transplant patients, BAL in conjunction with transbronchial biopsy is used to distinguish rejection from infection (13). However, BAL alone is not sufficient to have the diagnosis of rejection. Finally, BAL is also used for therapeutic removal of mucus plugs, blood clots and bronchial casts (14).
Whole lung lavage is another method of obtaining samples from lung and also therapeutic in pulmonary alveolar proteinosis and few other conditions. It rarely indicates in children and requires partial cardiopulmonary bypass or single lung ventilation.
Technique of BAL
BAL is effectively performed during FB. During the procedure, contamination of lower airway specimen with upper airway secretions should be avoided. Suction of the fluid should be done after the tip of the bronchoscope is passed through the most distal airway. Before the FB, selection of the site is decided based on clinical, radiologic and bronchoscopic findings. FB is directed to selected lobe and BAL is obtained from that lobe initially. If there is diffuse disease, BAL can be obtained from multiple lobes especially from lingual and right middle (5). After wedged to selected lobe, sterile saline is installed through suction channel and 1–2 mL of air is installed to clear the saline from the channel after each aliquot. During the suction of saline, extensive negative pressure should be avoided to overcome alveolar collapse. This causes not only insufficient sampling but also alveolar trauma.
The temperature of saline can be warmed up to body temperature (37 °C) or keep in room temperature. There is no consensus about the number and volume of aliquots that used in BAL. In adults, 3 aliquots with 100 mL or 5 aliquots with 50 mL is recommended (15). Various protocols have been developed for children. Some of the bronchoscopists use standard volume of 10 to 20 mL in 2 to 4 aliquots regardless of the weight and age of the children. Some others adjust the volume of the aliquots based on body weight. Ratjen et al. suggested that 3 mL/kg of sample into 3 aliquots with maximum volume of 20 mL/kg can be used in children (16). In general, 40–60% of installed fluid is recovered and the remainder will be absorbed. The first aliquot is relatively rich in fluid from the surface of the conducting airways and may have higher percentage of inflammatory cells. This sample can be used for cell count whereas remainder samples can be reserved for microbiological analysis. BAL sample fluid should be processed rapidly (less than 4 h) or keep at 4 °C until time to processing. Microbiologic studies including simple stains and special stains can be performed. Polymerase chain reaction (PCR) can be used to identify the pathogens and cytologic analysis including total cell counts, flow cytometry and lymphocyte subsets can be investigated.
Interpretation of BAL findings
Normal BAL fluids contain less than 5% of neutrophils and neutrophil counts can be detected up to 95% in bacterial infections (17). Less than 25% of neutrophil count is rarely indicates bacterial infection. Increased neutrophil counts can be associated with aspiration, asthma, cystic fibrosis, acute respiratory disease and alveolitis. Alveolar macrophages are most common non-epithelial cells in BAL fluids and constitutes 80–90% of cell counts. Lymphocytes are the second most common cells and composing 5–10% of total cells. Increased lymphocyte counts are not specific to a disease but significantly higher in sarcoidosis, M. tuberculosis infection, interstitial lung disease, hypersensitivity pneumonitis, Pneumocystis jiroveci infection and non-tuberculous mycobacterial infection (5). Eosinophils are rare in healthy children (0–1%) and higher in allergic and parasitic diseases. Pneumocystis carinii infection, interstitial lung disease and drug-induced lung disease also had elevated eosinophil counts in BAL samples (18).
Epithelial cells are common in BAL fluids. Squamous cells are from upper airways whereas ciliated columnar cells are from lower airways. Staphylococcus aureus, Haemophilus influenza and Streptococcus pneumonia with a concentration of more than 100,000 organism/mL of BAL fluid in association with elevated neutrophils are considered as evidence of infection. Absence of neutrophils, bacteria in BAL liquids present contamination rather than infection. However, density of bacteria more than 500,000 organisms/mL is considered as bacterial infection (5). Multiorganisms can be isolated from BAL of children with aspiration. Also, pathogens that are not normally seen in lungs, considered as infection regardless of numbers in immunocompromised children.
Therapeutic indications
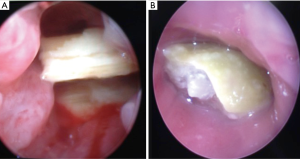
Most of the therapeutic indications of bronchoscopy in children are restoration of airway patency. Although FB can be used for these indications, RB is superior to FB to remove foreign bodies and other interventional procedures. Foreign body removals are difficult and potentially dangerous. Favorable results were also reported with FB in small and peripherally located foreign bodies (19). However, it can be still difficult with FB. RB has several advantages such as requiring general anesthesia, assisted ventilation, larger instruments and greater variety of forceps (3). Foreign body aspiration may be seen with variable presentation ranging from subtle cough or recurrent pneumonia to a sudden fatal asphyxia. Diagnosis can be obtained by history, radiologic findings and physical examination. Most of the foreign bodies are non-radiopaque but some of them can be easily seen in chest X-rays (Figure 2). When clinical suspicion is achieved, bronchoscopy is indicated regardless of negative radiologic and clinical findings. Negative exploration rate is reported as 10-15% in several series (1). Foreign bodies can be easily detected and removed by use of RB with the help of ventilation-assisted technique (Figure 3).
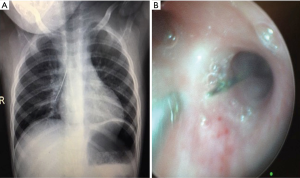
Despite from foreign bodies, mass lesions such as granulation tissue and tumor lesions can be removed or biopsied with RB. Granulation tissue is the most common lesion result from foreign bodies, mycobacterial infection and mechanical trauma due to artificial airways. Also mucous blood clots are causes of atelectasis and can be removed with FB. Plastic bronchitis is a rare disorder characterized by formation of bronchial casts in the tracheobronchial tree with partial or complete airway obstruction. Serial RB is needed when plastic bronchitis is unresponsive to medical treatment or cause airway obstruction (20).
Mass lesions are rare in children. Bronchial carcinoids, hemangiomas and inflammatory myofibroblastic tumors can be seen during childhood (21). Benign and polypoid lesions can be resected with forceps and laser by the help of RB. Malignant tumors usually extend to bronchial wall and cannot be resected with bronchoscope. Transbronchial biopsy (TB) is available for histopathological diagnosis. TB is a potential bleeding procedure and RB has advantage for bleeding control over FB. TB is useful to evaluate the lung parenchyma and avoids need for thoracotomy. It can be especially carried out in patients with lung transplant with a sensitivity of 72–94% and specificity of 90–100% (3). However, it is not recommended for the diagnosis of idiopathic interstitial pneumonopathies in children in which open biopsy is indicated (22). Lasers with near-infrared and visible light spectrum are also used for the management of both benign and malign lesions (5).
Tracheal and bronchial stenosis and severe malacia can be treated with the help of bronchoscopy. According to the nature of lesion, they can be dilated, lasered or stented (23,24). FB is also valuable in the management of difficult or complicated intubation. Intractable air leaks can be treated with Fogarty catheter, saline, fibrin glue or gel foam application.
Bronchoscopy can be done in conjunction with the surgery (25). Such as:
- To define position and cannulation of congenital or acquired tracheoesophageal fistulae;
- To define the effect of surgical manipulations intraoperatively during aortopexy and tracheopexy;
- To have selective bronchogram in case of localized bronchiectasis;
- Inspection of airways during the surgery.
Which type of bronchoscopy?
One of the most important issues is to define the type of bronchoscopy due to appropriate indication. Bronchoscopist should decide to use FB or RB to have the accurate diagnosis or to perform the best therapeutic intervention. Table 4 summarizes the indications and options of bronchoscope types for each clinical problem.

Full table
Contraindications to bronchoscopy
There are no absolute contraindications for bronchoscopy; however, suitable indication, appropriate equipment and skilled personnel are mandatory to perform a safe procedure. Relative risk factors are cardiovascular instability, bleeding diathesis (thrombocytopenia or hypoproteinemia), severe bronchospasm and hypoxemia (5). Some of indications such as severe airway obstruction may increase the risk of complications.
Complications of bronchoscopy
Both FB and RB are safe procedures and complications are related with the patient’s risk factors. Complications of RB are also due to instrumentation (bronchoscope itself), medication used and ventilation technique. Main complications are (3):
- Nasal trauma and epistaxis;
- Desaturation and hypoxemia;
- Cough and bronchospasm;
- Trauma and obstruction of airway due to edema;
- Hemorrhage;
- Pneumothorax;
- Fever and infections.
Fever can be seen 15% of patients especially BAL had been carried out during the procedure. It is related with cytokine release or with the transitory bacteremia.
Conclusions
In children, the most common indications for bronchoscopy are recurrent croup, chronic stridor and suspected foreign body. Bronchoscopy allows for examination of airway anatomy and dynamics. One of the most important role of bronchoscopy in the diagnosis of airway disease is obtaining samples from the airways. BAL samples are commonly used for differential diagnosis of several pulmonary problems. BAL findings should be carefully evaluated for the differential diagnosis. In addition to its diagnostic aid, bronchoscopy can be used for therapeutic indications. According to the indications and patient’s needs, appropriate type of bronchoscopy (FB or RB) should be chosen.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Jacobs IN. Bronchoscopy. In: Mattei P. editor. Fundamentals of Pediatric Surgery. New York: Springer, 2011; 185-94.
- Sinha V, Gurnani D, Barot DA. A study of applications of rigid bronchoscopy in pediatric patients. Indian J Otolaryngol Head Neck Surg 2014;66:142-4. [Crossref] [PubMed]
- Pérez-Frías J, Moreno Galdó A, Pérez Ruiz E, et al. Pediatric bronchoscopy guidelines. Arch Bronconeumol 2011;47:350-60. [Crossref] [PubMed]
- Bailey AG, Valley RD, Azizkhan RG, et al. Anaesthetic management of infants requiring endobronchial argon laser surgery. Can J Anaesth 1992;39:590-3. [Crossref] [PubMed]
- Wood RE, Daines C. Bronchscopy and bronchoalveolar lavage in pediatric patients. In: Wilmott RW, Bush A, Boat TF. editors. Kending and Chernick’s Disorders of the Respiratory Tract in Children. Eight edition. Philadelphia: Saunders, 2012; 94-109.
- Masters IB, Cooper P. Paediatric flexible bronchoscopy. J Paediatr Child Health 2002;38:555-9. [Crossref] [PubMed]
- Hitter A, Karkas A, Schmerber S, et al. Rigid bronchoscopy. In: Prifitis KN, Anthracopoulus MB, Eber E, et al. editors. Pediatric Bronchoscopy: Progress in Respiratory Research. Basel: Karger, 2010;38:83-94.
- Boudewyns A, Claes J, Van de Heyning P. Clinical practice: an approach to stridor in infants and children. Eur J Pediatr 2010;169:135-41. [Crossref] [PubMed]
- Woods RK, Sharp RJ, Holcomb GW 3rd, et al. Vascular anomalies and tracheoesophageal compression: a single institution's 25-year experience. Ann Thorac Surg 2001;72:434-8; discussion 438-9. [Crossref] [PubMed]
- Wood RE, Prakash UB. Pediatric flexible bronchoscopy. In: Praksh UB. editor. Bronchoscope. New York: Raven Press, 1994; 345-56.
- Balough K, McCubbin M, Weinberger M, et al. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol 1995;20:63-70. [Crossref] [PubMed]
- Colombo JL, Hallberg TK. Recurrent aspiration in children: lipid-laden alveolar macrophage quantitation. Pediatr Pulmonol 1987;3:86-9. [Crossref] [PubMed]
- Belperio JA, Weight SS, Fishbein MC, et al. Chronic lung allograft rejection. Proc Am Thorac Soc 2009;6:108-21. [Crossref] [PubMed]
- McKenzie B, Wood RE, Bailey A. Airway management for unilateral lung lavage in children. Anesthesiology 1989;70:550-3. [Crossref] [PubMed]
- Reynolds HY. Bronchoalveolar lavage. Am Rev Respir Dis 1987;135:250-63. [PubMed]
- Ratjen F, Bruch J. Adjustment of bronchoalveolar lavage volume to body weight in children. Pediatr Pulmonol 1996;21:184-8. [Crossref] [PubMed]
- Riedler J, Grigg J, Stone C, et al. Bronchoalveolar lavage cellularity in healthy children. Am J Respir Crit Care Med 1995;152:163-8. [Crossref] [PubMed]
- Oermann CM, Panesar KS, Langston C, et al. Pulmonary infiltrates with eosinophilia syndromes in children. J Pediatr 2000;136:351-8. [Crossref] [PubMed]
- Tang LF, Xu YC, Wang YS, et al. Airway foreign body removal by flexible bronchoscopy: experience with 1027 children during 2000-2008. World J Pediatr 2009;5:191-5. [Crossref] [PubMed]
- Soyer T, Yalcin Ş, Emiralioğlu N, et al. Use of serial rigid bronchoscopy in the treatment of plastic bronchitis in children. J Pediatr Surg 2016;51:1640-3. [Crossref] [PubMed]
- Karnak I, Haliloğlu M, Orhan D, et al. Pure endobronchial inflammatory myofibroblastic tumor in children. J Pediatr Hematol Oncol 2014;36:108-10. [Crossref] [PubMed]
- Fan LL, Kozinetz CA, Wojtczak HA, et al. Diagnostic value of transbronchial, thoracoscopic, and open lung biopsy in immunocompetent children with chronic interstitial lung disease. J Pediatr 1997;131:565-9. [Crossref] [PubMed]
- Bagwell CE, Talbert JL, Tepas JJ 3rd. Balloon dilatation of long-segment tracheal stenoses. J Pediatr Surg 1991;26:153-9. [Crossref] [PubMed]
- Antón-Pacheco JL, Cabezalí D, Tejedor R, et al. The role of airway stenting in pediatric tracheobronchial obstruction. Eur J Cardiothorac Surg 2008;33:1069-75. [Crossref] [PubMed]
- Donato LL, Mai Hong Tran T, Ammouche C, et al. Pediatric interventional bronchoscopy. Clin Chest Med 2013;34:569-82. [Crossref] [PubMed]