A novel surgical method for acquired non-malignant complicated tracheoesophageal and bronchial-gastric stump fistula: the “double patch” technique
Introduction
Acquired non-malignant fistula between the airway and upper gastrointestinal tract is a rare but challenging clinical issue (1). Patients with this condition have little chance of self-recovery. Abnormal communications between the airway and upper gastrointestinal tract lead to continuous contamination of the tracheobronchial tree and repeated respiratory infections. In the absence of timely intervention, patients may die of respiratory failure or malnutrition within 3–4 months (2,3). The etiology of the fistula includes complications of mechanical ventilation, indwelling on tracheal or esophageal stents, complications from prior tracheal or esophageal surgery, granulomatous mediastinal infections, trauma, iatrogenic injuries, and caustic ingestion (1). Acquired non-malignant complicated tracheoesophageal fistula (TEF) and bronchial-gastric stump fistula (BGSF) are characterized by a large fistula (≥1 cm), severe inflammation, high rate of postoperative death and recurrence, hard to perform stent placement, need for segmental tracheal or bronchial resection, or primary anastomosis of esophageal and tracheal defect that cannot been surgically repaired (4,5).
Different approaches have been proposed to treat the acquired non-malignant TEF and BGSF. The currently available treatments include minimally invasive procedures and surgeries. Previous studies have shown varying levels of success in the use of minimally invasive procedures in patients with early detection, small fistula (<1 cm), and mild local infection. Some of these methods were covered stent placement, cardiac septal occlusion, chemical cautery, injection of Bioplastique®, histoacryl glue, glutaraldehyde crosslinked (GAX) collagen, calcium hydroxylapatite (CaHA), and Tisseel® fibrin glue (6-12). The minimally invasive procedures are advantageous because of ease of handling, simplicity, and small trauma; however, they have disadvantages of increased postoperative complications and high recurrence rate (6,13,14). The respiratory tract resection combined with end-to-end anastomosis is suggested by some surgeons to treat patients with complicated fistula (≥1 cm), severe local infection, and tissue edema. However, this method has the following limitations: time-consuming and complex procedure, increased surgical trauma, complications, and high mortality. Moreover, the length of the respiratory tract that can be removed is limited, which adds to the disadvantages of the surgery. Although it is an alternative to the complex surgical procedure of respiratory tract resection, using autologous muscle flap, pericardium, allogeneic pericardium, and other materials (AlloDerm patch) as the flap to repair the fistula is associated with disadvantages such as insufficient blood supply, limited available material for the flap repair, allograft rejection, high recurrence rate, and the need for separating surrounding tissues from the fistula (15,16). Previous studies have reported that using a neo-membranous airway with the single-layer posterior esophageal wall or simultaneously using a pedicled muscle flap to repair the fistula is capable of closing the large TEF (17-19). This method has some advantages such as the flap can be obtained locally, there is no allograft rejection, separation of the surrounding tissues from the fistula is not required, removal of the respiratory tract is not necessary, and a muscle flap can resist the high pressure in the airway. However, for patients with a large fistula, severe local infection, tissue edema, and fragile tissues around the fistula, the single-layer esophageal flap only may not resist the high pressure in the airway, which will lead to the recurrence of fistula and tracheal stenosis (17). In addition, using a pedicled muscle flap to resist the high pressure in the airway is a complicated procedure, for which the availability of material is limited, and the blood supply cannot be certain.
Whether the acquired non-malignant complicated TEF and BGSF can be closed safely and efficiently without a pedicled muscle flap, requires further investigation. Herein, we described a novel “double patch” technique for the repair of the trachea, which uses the neomembranous esophageal walls of double layers to close the defect of the trachea or bronchus (4). We retrospectively reviewed and assessed the long-term outcomes of 30 patients with acquired benign complicated TEF and BGSF at our hospital between August 2004 and August 2014.
Methods
Clinical data
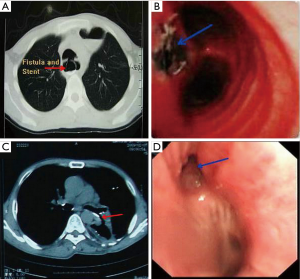
We reviewed the medical records of all patients with TEF and BGSF in the Department of Thoracic Surgery, Tangdu Hospital Affiliated to the Fourth Military Medical University, Xi’an, Shaanxi, China between August 2004 and August 2014. The duration of follow-up was 24 months. Diagnosis of the TEF and BGSF were established using upper gastrointestinal endoscopy, bronchoscopy, or upper gastrointestinal barium series. Patients who were suspected with a fistula on a computed tomography (CT) scanning of the chest were excluded if the fistula was not proven by one of the methods mentioned above (2). Patients with malignant and congenital TEF were excluded from the present study. Finally, 30 patients who underwent surgical management for acquired benign TEF and BGSF during this span (Figure 1) were included in the survey. The length of defects on the membranous region of the trachea and bronchus ranged from 1–5 cm. The baseline characteristics of 30 patients were recorded (Table 1). The study protocol was approved by the Regional Ethics Committee for Clinical Research of the Fourth Military Medical University (Number/ID: TDLL-201512-019).
Full table
Operative techniques
The surgical procedure for all the patients was performed by an identical team, which included a senior thoracic surgeon, an anesthetist, and two junior thoracic surgeons. An endotracheal tube for ventilation was inserted before surgery. When the defect was located on the main trachea or up the arch of azygos vein, the double-lumen endotracheal tube balloon was sent into the main bronchus to ensure ventilation, and a posterolateral thoracotomy was performed through the right 4th or 5th intercostal space. In the case of the defect located on the main bronchus, the double-lumen endotracheal tube balloon was sent into the contralateral main bronchus to ensure ventilation, and a posterolateral thoracotomy was performed through the fifth intercostal space or the prior thoracic incision.
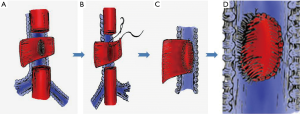
The esophagus or gastric stump was not separated from the fistula. The thoracic segment of the esophagus was transected away from the proximal and distal edge of the fistula to approximately 1–2 cm. Then, a longitudinal incision was made to split open the esophageal lumen along the longitudinal axis of the esophageal posterior wall away from the left edge of the fistula by approximately 2–2.5 cm. Two esophageal patches were formed, a long one and a short one, on either side of the fistula (Figure 2A) (4). Subsequently, the mucosa of the patches was cauterized. The short patch was sutured to the edge of the fistula with 3-0 absorbable interrupted full-thickness sutures, which closed and buttressed the fistula completely (Figure 2B,C). The short patch was covered with the long patch (Figures 2D). Based on the same principle, a conformal incision was made on the wall of the gastric stump to form two patches similar to that of the BGSF: above and below the fistula (Figure 3A,B). Subsequently, the mucosa of the patches was cauterized. The short patch was sutured to the edge of the fistula with 3-0 absorbable interrupted full-thickness sutures, which closed the fistula completely (Figure 3C,D). The short patch was covered with the long patch (Figure 3E).
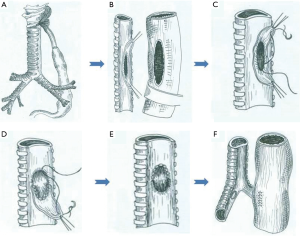
In the same stage, the stomach was mobilized through the opened diaphragmatic hiatus or by laparotomy, preserving the right gastroepiploic vessels and the epiploic arcade. Subsequently, the stomach was tubed and pulled up into the thorax, and an esophagogastric anastomosis was performed with a tubular anastomat. Alternatively, gastrorrhaphy was conducted (Figure 3F). The chest was closed after insertion of two intercostal drains.
Results
The duration of follow-up was 24 months, and no mortality was observed in this cohort of 30 patients. All of them finally resumed oral intake.
Twenty-six patients (86.7%) who underwent the double patch technique showed complete healing, without significant complications occurring in the postoperative period. The success of the repair was examined using a bronchoscope; no remarkable swelling of the patch and stenosis was noted (Figure 4).
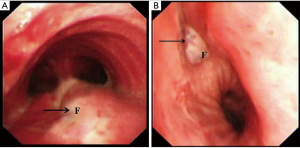
In one patient (3.3%), with the recurrence of tracheal fistula (3.5 cm) in situ at postoperative 2nd week, tracheal intubation was performed for assisting mechanical ventilation, and nasogastric nutrition was operated immediately. The patient was managed conservatively. The patient was treated with tracheal resection, and the tracheal continuity was maintained with end-to-end anastomosis when the physical condition reached surgical requirements. Bronchoscopy during the follow-up showed complete healing of the fistula.
Two patients (6.7%) presented complication (pneumonia) in the perioperative and postoperative period, which was mainly caused by TEF and BGSF. Both the patients were managed conservatively and were administered antibiotics and bronchoscopic suctioning. They were treated for 10 days, and pneumonia was cured completely.
Failure to resume oral intake in one patient (3.3%) was due to fistula esophageal anastomosis, which contributed to empyema. Nasogastric nutrition and closed thoracic drainage were performed immediately. The patient was managed conservatively and treated for 1 month. Esophagoscopy showed complete healing of the esophageal fistula, and oral intake was restored immediately.
Discussion
The present study aimed to retrospectively summarize and analyze patients over a longer follow-up period than a previous study (4), in order to verify that “double patch” technique was an effective and safe surgical method to treat non-malignant complicated TEF and BGSF.
Herein, we overlapped neo-membranous esophageal walls of double layers to repair the airway fistula. This technique created a firm and mucosa-free patch to resist the high pressure in airway without the reinforcement of the pedicled muscle flap. Any significant swelling of the tracheal patch and stenosis was not observed in the postoperative examination by bronchoscopy. Thus, our results provided strong evidence that “double patch” technique is a reliable method to treat acquired non-malignant complicated TEF and BGSF.
In this retrospective study, thirty patients who underwent the double patch technique were enrolled. Twenty-six patients showed complete healing and did not present any major complications in the postoperative period. However, one patient showed the recurrence of fistula in situ at postoperative 2nd week. One explanation may be that the esophageal blood supply may be disturbed and destroyed. Because of patient suffering from caustic ingestion may contribute to esophageal stenosis, and using esophageal stent for more than 6 months led to TEF, which may lead to insufficient blood supply for the double patches. Consequently, the patient was at a risk of ischemic necrosis and recurrence of fistula. Hence, for patients with insufficient blood supply of the esophagus, the length of double patches should be sufficient (away from the proximal and distal edge of the fistula to approximately 2–2.5 cm), and the cauterization should not destroy the esophageal muscular layer, thereby avoiding damage to the blood supply.
The gland in esophageal mucosa and submucosa can secrete a large amount of mucoprotein, which resists hydrogen ion and pepsin (20). In this study, the esophageal mucosa was cauterized, which might avoid the mucoprotein secretion by the mucosal gland. Because of a large amount of mucoprotein accumulated between the two patches may constrict the airway, which may contribute to stenosis of the airway. Therefore, the esophageal mucosal gland should be completely removed in the “double patch” technique. However, this piece of evidence needs to be investigated and proved further.
Additionally, based on the results of our previous study (4) and the present study, the “double patch” technique exhibit the following features: (I) there is no need to separate the fistula; (II) the part of the esophagus with the defect could be used to repair the fistula; (III) the tracheal defect could be repaired with an esophagus segment without mucosa; (IV) the esophageal segment adhering to the fistula should be tighter; (V) a pedicled muscle flap is not required in this technique as the tracheal wall is reinforced with two patches to resist the airway pressure; (VI) reconstruction of the digestive conduit is necessary; and (VII) there is a low risk of recurrence.
Importantly, some of the procedure-related challenges, such as morphological and pathophysiological changes, structural as well as functional changes of double patches, and the postoperative effect of with or without mucosa of patches on the “double patch” technique remain unclear. Thus, animal models are essential to investigate these challenges, which will lay the theoretical foundation of the technique.
Conclusions
In summary, the “double patch” technique is a safe and effective method to repair the acquired non-malignant complicated TEF and BGSF.
Acknowledgements
This work was supported by Dr. Qishu Cheng from the Department of Thoracic Surgery, Tangdu Hospital, the Fourth Military Medical University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Regional Ethics Committee for Clinical Research of the Fourth Military Medical University (Number/ID: TDLL-201512-019).
References
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8; discussion 919. [Crossref] [PubMed]
- Chen YH, Li SH, Chiu YC, et al. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One 2012;7:e42766. [Crossref] [PubMed]
- Hu Y, Zhao YF, Chen LQ, et al. Comparative study of different treatments for malignant tracheoesophageal/bronchoesophageal fistulae. Dis Esophagus 2009;22:526-31. [Crossref] [PubMed]
- Han Y, Liu K, Li X, et al. Repair of massive stent-induced tracheoesophageal fistula. J Thorac Cardiovasc Surg 2009;137:813-7. [Crossref] [PubMed]
- He J, Chen M, Shao W, et al. Surgical Management Of 3 Cases With Huge Tracheoesophageal Fistula With Esophagus Segment in situ As Replacement Of The Posterior Membranous Wall Of The Trachea. J Thorac Dis 2009;1:39-45. [PubMed]
- Kasbekar AV, Sherman IW. Closure of minor tracheoesophageal fistulae with calcium hydroxlapatite. Auris Nasus Larynx 2013;40:491-2. [Crossref] [PubMed]
- Lopes MF, Catré D, Reis A, et al. Endoscopic treatment of recurrent tracheoesophageal fistula with histoacryl glue. Gastrointest Endosc 2010;72:1324-5; author reply 1325. [Crossref] [PubMed]
- Remacle MJ, Declaye XJ. Gax-collagen injection to correct an enlarged tracheoesophageal fistula for a vocal prosthesis. Laryngoscope 1988;98:1350-2. [PubMed]
- Repici A, Presbitero P, Carlino A, et al. First human case of esophagus-tracheal fistula closure by using a cardiac septal occluder (with video). Gastrointest Endosc 2010;71:867-9. [Crossref] [PubMed]
- Rokade AV, Mathews J, Reddy KT. Tissue augmentation using Bioplastique as a treatment of leakage around a Provox 2 voice prosthesis. J Laryngol Otol 2003;117:80-2. [Crossref] [PubMed]
- Wang CY, Chou CH, Wang HP, et al. Successful treatment of bronchoesophageal fistula with esophageal and bronchial stenting. J Formos Med Assoc 2011;110:270-2. [Crossref] [PubMed]
- Wiseman NE. Endoscopic closure of recurrent tracheoesophageal fistula using Tisseel. J Pediatr Surg 1995;30:1236-7. [Crossref] [PubMed]
- DeFatta RA, Chowdhury FR, Sataloff RT. Complications of injection laryngoplasty using calcium hydroxylapatite. J Voice 2012;26:614-8. [Crossref] [PubMed]
- Halpern BS, Britz-Cunningham SH, Kim CK. Intense focal F-18 FDG uptake in vocal cord associated with injection of calcium hydroxylapatite microspheres. Clin Nucl Med 2011;36:e175-7. [Crossref] [PubMed]
- Golash V. Single-stage repair of a large acquired tracheoesophageal fistula with interposition of 2 muscle pedicle flaps and laparoscopic gastrojejunostomy. J Thorac Cardiovasc Surg 2006;131:1413-4. [Crossref] [PubMed]
- Su JW, Mason DP, Murthy SC, et al. Closure of a large tracheoesophageal fistula using AlloDerm. J Thorac Cardiovasc Surg 2008;135:706-7. [Crossref] [PubMed]
- Deshpande G, Samarasam I, Banerjee S, et al. Benign esophagorespiratory fistula: a case series and a novel technique of definitive management. Dis Esophagus 2013;26:141-7. [Crossref] [PubMed]
- Jougon J, Couraud L. Esophageal patching for an unsuturable tracheoesophageal fistula. Eur J Cardiothorac Surg 1998;14:431-3. [Crossref] [PubMed]
- Kalkat MS, Parmar JM, Collins FJ. Management of giant acquired tracheo-oesophageal fistula in a neonate using an oesophageal patch. Interact Cardiovasc Thorac Surg 2003;2:633-5. [Crossref] [PubMed]
- Namiot Z, Sarosiek J, Marcinkiewicz M, et al. Declined human esophageal mucin secretion in patients with severe reflux esophagitis. Dig Dis Sci 1994;39:2523-9. [Crossref] [PubMed]


