Real-world asthma management with inhaler devices in Switzerland—results of the asthma survey
Introduction
Asthma belongs to the most common respiratory diseases affecting 5% to 8% of the Swiss population (1). Asthma is an obstructive respiratory disease characterized by chronic inflammation of the airways with airway hyper-responsiveness, recurrent symptoms and exacerbations that vary over time and in intensity, and typically reversible expiratory airflow limitation (2). As recommended in the guidelines of the Global Initiative for Asthma, the goal of asthma treatment is to achieve asthma control, i.e., to control symptoms and risk factors for future poor outcomes (2). Inhaled administration of pharmaceutically active substances is the preferred route because it optimizes drug delivery directly to the airways as well as tolerability by minimizing systemic exposure. However, the capability of using the inhaler device appropriately is often insufficient, even in experienced outpatients (3,4), and is associated with poorer disease control and increased use of healthcare resources (5).
Proper inhaler technique is crucial for effective management of asthma and results from the complex interaction between the health care professionals, the therapy itself [i.e., the drug(s) and the inhaler device], and the patient in his environment. Health care professionals should ensure that the patient has acquired and maintains a correct inhaler technique over time (6,7). Patient education provided by health care professionals (physicians, but also pharmacists or nurses) with regard to the disease and to handling of the device has repeatedly been shown to be of crucial importance (8-10). While drug choice is usually the first step when prescribing an inhaled therapy, some have argued that choice of the inhaler device may be even more important (11,12). The available inhaler devices share common potential sources of handling errors. In addition, pressurized metered-dose inhalers (pMDI) and dry-powder inhalers (DPI) have specific requirements that may lead to additional errors in daily practice (7,13,14). Finally, the patient should ultimately be adherent and compliant with the prescribed therapy, which can be best achieved through better understanding of his medical history and comorbidities, cultural environment, health behaviors, and preferences (15).
The aim of the Asthma Survey was to generate insights about the daily practice of physicians with regard to inhaler devices used for treating asthma, under real-world conditions in Switzerland.
Methods
A questionnaire was designed and administered as 1:1 interviews to hospital- and practice-based Swiss physicians willing to participate by well-trained representatives of a pharmaceutical company operating in the field of obstructive respiratory diseases (Mundipharma Medical Company, Basel, Switzerland). All participating physicians were listed in the Swiss register of practicing physicians of the Swiss Medical Association (FMH) used by the sponsor (www.doctorfmh.ch). As shown in the Supplementary, in addition to physician demographics [age, specialty, canton of practice, and prescriber status (self-dispensing or not)], the predefined areas of interest were (I) aspects of patient education regarding the use of an inhaler device (three questions); (II) typical difficulties encountered in daily practice when prescribing a pMDI and DPI (two questions); (III) behaviors and real-world reasons for physician preferences in daily practice (four questions).
For the purpose of statistical analysis, descriptive methods were used. All physicians with known place of practice were included in the analysis. For unprompted answers (free text), category headings were defined post hoc and adjudication performed by an informed physician. In the primary analysis, overall means with corresponding standard deviations or medians with interquartile ranges were calculated for normally and non-normally distributed variables, respectively. In a secondary analysis, differences between linguistic regions [German-speaking part of Switzerland (D-CH) and French- and Italian-speaking parts of Switzerland (W-CH) parts of Switzerland] were tested for statistical significance by exploratory chi-square tests and by calculating exploratory odds-ratios with corresponding 95% confidence intervals using the statistical software SPSS version 19.1. A P value below 0.05 was considered statistically significant.
The survey was performed in accordance with the Helsinki Declaration as revised in 2013. Formal Ethical Review Committee approval was not necessary per written declaration of no ethical impediment Nb. 77-2015 of the cantonal ethical commission of Zurich, Switzerland.
Results
Data collection occurred between April 2014 and August 2015. In total, 605 physicians accepted to participate. Of these, 529 (87.4%) provided datasets suitable for analysis, 291 (55.0%) in the D-CH and 238 (45.0%) in the W-CH part of Switzerland. The final dataset included questionnaires from 342 internists/general practitioners (190 and 152 in D-CH and W-CH, respectively), 177 pulmonologists/allergologists (95 and 82), and 10 other (6 and 4). The response rate was 34.8% and 6.2% of all hospital- or private practice-based pulmonologists/allergologists and general practitioners/internists in Switzerland, respectively. Self-dispensing physicians represented 56.0% of all included in D-CH and prescribing physicians 90.3% of all included in W-CH.
When a new fixed combination of an inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) was prescribed to a patient with asthma, prior therapy was either another ICS + LABA (49.1%), a short acting beta-agonist (SABA, 38.1%), an ICS monotherapy (20.7%) or a leukotriene receptor antagonist (LTRA, 8.3%) with multiple answers allowed. A direct switch from a SABA to a fixed ICS + LABA combination was significantly more likely to occur in D-CH compared to W-CH (44.2% vs. 30.7%; OR =1.7; 95% CI, 1.1–2.5; P=0.007). Conversely, a switch from another ICS + LABA was significantly less likely to occur in D-CH (39.9% vs. 60.2%; OR =0.4; 95% CI, 0.3–0.6; P<0.001).
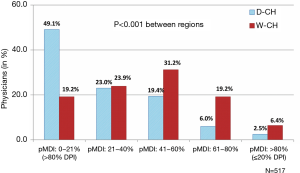
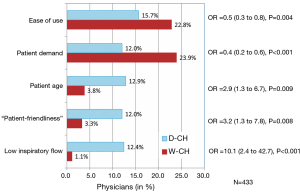
DPI devices were overwhelmingly preferred over pMDI, with 83.8% of the physicians prescribing DPI to 40% or more of their patients. At the extremes, when considering the inhaler type prescribed to more than 80% of their patients, 35.6% of the physicians favored a DPI and only 4.3% a pMDI (P<0.001). Interestingly, the prescription pattern was different between regions, with significantly more pMDIs prescribed in W-CH than in D-CH (Figure 1). The main cited reasons for prescribing a pMDI were ease of use (18.7%), patient demand (17.7%), expected better drug deposition (17.1%), less side-effects (10.2%), patient age (9.0%), “patient-friendliness” (8.3%), and low inspiratory flow requirement (7.6%). As shown in Figure 2, ease of use and patient demand were significant drivers of pMDI preference in W-CH while patient age, patient-friendliness, and low inspiratory flow were considered more important in D-CH.
The most common errors (occurring frequently or occasionally) encountered in daily practice when using a DPI were an insufficiently forceful inhalation (88.8%), insufficient breath-holding after inhalation (83.2%), and the absence of expiration before inhalation (80.9%). Additional errors occurred with device actuation (59.2%) and through expiration in the actuated device (43.7%). The most common errors reported when using a pMDI were poor coordination between device actuation and inhalation (90.5%), insufficient breath-holding after inhalation (79.6%), the absence of expiration before inhalation (76.2%). Additional errors were related to excessively fast inhalation (75.4%) and to wrong device actuation (39.3%). Differences between regions were not statistically significant.
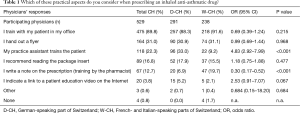
As shown in Table 1, approximately 90% of all Swiss physicians declared being personally involved in educating their asthma patients using an inhaler device (88.3% vs. 92.0% in D-CH and W-CH, respectively). Of those who implemented patient education and inhaler training themselves (n=475), most made usage of a second education layer by either handing out a descriptive flyer (31.2%), requesting additional training by their practice assistant (17.5%), recommending to read the package insert (16.4%), directing the patient to the pharmacist for training (12.0%) or indicating other external resources such as a Web link (4.6%). The physicians who did not educate their patients themselves (n=54), delegated inhaler training to either their practice assistant (64.8%) or the pharmacist (18.5%) and/or distributed a handout (29.6%) or recommended reading the package insert (20.4%). Only 7.4% did not implement any patient education measures. Differences between regions were not statistically significant, with the exception of involving the practice assistant (more frequent in D-CH, 33.0% vs. 9.2%; OR =4.8; 95% CI, 2.9–8.0; P<0.001) and involving the pharmacist (less frequent in D-CH, 6.9% vs. 19.7%; OR =0.3; 95% CI, 0.2–0.5; P<0.001).
Full table
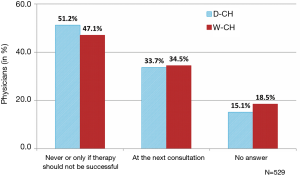
Patient skills with regard to inhalation technique and effective inhaler handling were generally not monitored on a regular basis (Figure 3). Only 34.0% of all participating physicians declared systematically checking inhalation technique at the next visit(s), i.e., 3 weeks (median value, IQR 2 to 4 weeks) after the first prescription or a prescription change and every 12 weeks thereafter (IQR 8 to 24). Half of the physicians (49.3%) declared never controlling the inhalation technique or only in cases in which therapy was unsuccessful. An additional 16.7% eluded the question. Differences across regions were not statistically significant.
Physicians were generally satisfied with the patient education tools at their disposal with 41.3% stating that they were missing nothing. However, some requested an improved access to demonstration devices (24.6%) and to didactic materials for patients (17.7%). Inhalation control systems, spacers, and single usage nozzles were considered missing by less than 5% of the physicians.
Finally, in this Asthma Survey, an average (mean ± SD) of 18 patients with any form of obstructive respiratory disease were seen per physician per week, of which 6.4±7.4 (35.7%) with asthma, 7.7±7.8 (42.5%) with chronic obstructive pulmonary disease (COPD), and 3.9±3.9 (21.8%) with ACOS. Differences between linguistic regions were not statistically significant.
Discussion
The Asthma Survey explored the level of education and inhaler training among physicians treating patients with asthma in Switzerland under real-world conditions of daily practice. Patient education seems widely accepted and implemented with strong involvement of the treating physician and regional differences in the roles of specific stakeholders. On the other hand, the monitoring over time of patient skills regarding appropriate use of their inhaler device has not become a generally implemented routine yet, indicating room for improvement in both regions. Within a context of clear preference for DPI devices in both regions, a surprising finding was that pMDIs were used more frequently in W-CH than in D-CH and that the reasons for preference differed by region. Finally, direct switches from SABA to a fixed combination of ICS+LABA, which constitute a deviation from the stepwise treatment approach recommended by GINA, were more frequent in D-CH. Overall, these findings indicate that within the same country, subtle differences in perceptions exist which may be relevant and should be further explored before considering the nationwide implementation of physician and patient education programs in the field of chronic respiratory diseases.
While more than 90% of the physicians declared being personally involved in educating their asthma patients, it should be remembered that an earlier Swiss survey involving 281 general practitioners and 1,893 patients with follow-up visits who undertook the Juniper Asthma Control Questionnaire, showed that only 16% were effectively controlled with a clear gap between the physician perceived level of asthma control and the objective measure (16). Furthermore, in another Swiss survey performed in the region of Zurich, Switzerland, only 24% of the participating physicians provided their patients with written action plans for self-management as recommended by the GINA guidelines (17). It is reasonable to assume that the high declared level of good will and personal involvement, which acknowledges the high perceived need for patient education, may suffer from the time constraints imposed to daily practice and contrast with achieved results. It is also likely that Swiss physicians almost systematically add the second layer of patient education/information reported in this survey as an anticipatory response to this perceived lack of efficiency. It has been repeatedly shown that health care providers (across all disciplines) often do not sufficiently master the inhalation technique themselves (7,11,18,19) and are not sufficiently aware of device-specific handling difficulties, even more so in an increasingly complex environment with a steadily growing inhaler diversity (11,13,20-22). In this context, the best person to provide inhaler training should be the one with the appropriate skills, adequate time, and access to teaching resources. This may be the physician himself or his practice assistant (as exemplified in D-CH) or the pharmacist (in W-CH). The regional difference in terms of educational resources involved may be related to the fact that most participating physicians in D-CH were self-dispensing, while most in W-CH were prescribing, which arguably increases the intensity level of interaction with the pharmacist.
In this Asthma Survey, only approximately one third of the physicians controlled inhaling skills and repeated the instructions at each follow-up visit, with virtually no difference between regions. This is of major concern. It has been shown that even patients who initially learned to use their inhaler device properly did not maintain the correct inhalation technique over time (7,23). It has also been shown that while verbal instruction combined with physical demonstration is the most effective training technique for correct inhaler use (7,23,24), only repeating this education does prevent drifts and increase the proportion of patients maintaining a correct inhalation technique over time (6,9). Internet-based tutorials for patients and physicians should be considered interesting complementary but not substitutive information sources in this regard (25,26). Thus, continuous medical education should focus on “training the trainer” regarding inhaler-specific knowledge and hands-on skills. It should also insist on the importance of requiring patients to demonstrate their inhaler technique at each follow-up visit.
Despite the fact that all aerosol drug delivery devices were shown in a systematic review to be equally efficacious in terms of outcomes, provided that they were used appropriately (27), a wide diversity in inhaler prescription exists between European countries (28). In years 2002–2008, pMDIs constituted over 70% of the inhalation devices sold in UK whereas they represented only approximately 10% in Sweden, with differences possibly related to differing health policies, costs, commercial aspects as well as prescribers' and patients' preferences (28). Based on the findings in this survey, Switzerland clearly is a country in which physicians prefer to prescribe DPIs. Interestingly, the occurrence of general inhaler handling errors (absence of expiration before inhalation and insufficient breath-holding after inspiration) was similarly high with both DPI and pMDI. The nature of DPI- and pMDI specific errors was different (typically too slow and insufficiently forceful inhalation with DPI and poor coordination and too fast inhalation with pMDI) but of similar order of magnitude, approximating 80–90% which is consistent with earlier findings showing that in the real-world at least some handling errors occur in 80% of the patients (20). In addition, no statistically significant difference in these error profiles existed across regions. Nevertheless, pMDI were used significantly more frequently in W-CH than in D-CH. Although this preference is consistent with a slightly higher use of pMDI in France than in in Germany (28), the reasons for this difference remain speculative. On one hand, in W-CH, ease of use and patient demand were significantly more important reasons for prescribing a pMDI than in D-CH. The fact that rescue asthma medication (SABA) is generally formulated as a pMDI may have been perceived as a contribution to the ease of use of pMDI in general, capitalizing on already trained and hopefully mastered skills. This is consistent with the recommendation not to switch between different inhalers, as each inhaler class and brand has its own unique requirements regarding handling and inhalation technique (29-31). In addition, patients do express preference for particular inhaler devices, which makes successful teaching easier (32,33). On the other hand, in D-CH, pMDI prescriptions were significantly more often triggered by patient age and by the presence of a low inspiratory flow than in W-CH, which is consistent with more technically objective reasons for prescribing and with the recommendation that choice of device should be based on the individual patient’s natural inhaler technique, preferring an MDI in patients who naturally tend to breathe in slowly and preferring a DPI in those who tend to inspire hard and fast (7,11). The Swiss healthcare system is identical in all linguistic regions, basically consisting of a compulsory health insurance which covers all costs related diagnostic and therapeutic measures, including for all chronic airway diseases. Differences between regions are therefore unlikely to be related to the healthcare system itself. It can be hypothesized that they may relate to human factors such as physician training (with two University Hospitals in the French-speaking part and three in the German-speaking part), patient preference, and last but maybe not least, the individual performance of sales representatives (who by nature differ by linguistic region).
It should be remembered that “the ideal inhaler does not exist in real life” (34). An ERS/ISAM consensus statement provided clear guidance for choosing the most appropriate aerosol delivery device based on a patient’s actuation-inhalation coordination skills and level of inspiratory flow, among other clinical conditions (22,34). The correct use of a pDMI requires adequate coordination between actuation of the device and inhalation (or the use of a spacer) which has been rendered obsolete with DPIs (3). With the ultimate goal of controlling symptoms and slowing or stopping disease progression, the intermediate goal with any inhaler device is to ensure drug delivery at the right dose in the right place. In this respect, DPIs require higher inspiratory flows than pMDIs to ensure optimal particle size and delivery to the lower airways. Optimal particle size is 2–5 µm with smaller particles reaching the alveoli and larger particles settling in the mouth, both increasing systemic exposure and limiting clinical effectiveness (35). With DPI devices, the inhalation flow rate and the device-specific DPI intrinsic resistance must be perfectly balanced to ensure optimal particle size and airway deposition (36). For similar drug delivery and efficacy, low-resistance DPIs require a higher inhalation flow rate than medium- or low-resistance DPIs, leading to a wide range of device-specific flow rates needed for optimal drug delivery. In the currently available DPI devices, the required flow rates range from 37 L/min with the Handihaler® to 111 L/min with the Breezhaler® (37). In other words, either a DPI device-specific optimization of the inspiratory flow rate or the selection of an appropriate DPI inhaler for a given inspiratory flow is needed for optimal efficacy. In any case, adequate device-specific patient education is required but often neglected when prescribing a DPI in daily practice.
Finally, in the present Asthma Survey, approximately 22% of the patients with obstructive respiratory disease were considered having a mixed form of asthma and COPD, consistent with the recently characterized asthma-COPD overlap syndrome (ACOS) (38,39). This figure is remarkably consistent with the reported prevalence rate of 15% to 25% of concurrent physician-diagnosed asthma and COPD (39-42). While a specific definition of ACOS is still lacking, due to poorly understood phenotypes and underlying mechanisms, it is characterized by persistent airflow limitation with several features usually associated with asthma and several others associated with COPD (39). It is well accepted that patients with features of both asthma and COPD experience more severe outcomes in terms of exacerbations, quality of life, decline in lung function, and mortality and use more healthcare resources than patients with either single disease alone (38,39,43). A stepwise diagnostic approach, as recently recommended (39), was obviously not part of the present survey, such that the true prevalence of ACOS cannot be inferred from the present findings. However, it is noteworthy that in a formerly dichotomic world in which asthma and COPD were separate clinical entities, practicing physicians clearly identify mixed forms of obstructive lung disease amongst their patients in similar proportions across linguistic regions. Further efforts should be undertaken to better understand whether these patients are appropriately diagnosed and get appropriate treatment.
The results of the present survey should be considered indicative of possible trends and serve as a basis for triggering further research. Among its strengths, the Asthma Survey collected real-world data under conditions of daily practice in a large sample of physicians. In addition, it is the first of its kind investigating differences between linguistic regions within a single country, highlighting the effects of cultural differences on daily medical practice. Heterogeneity across interviewers has been minimized by specific training prior to implementation. By nature, it reflects only the responses of physicians who accepted to participate, although the study sample was large. Most questions had a free text answering option. Although adjudication was done by a health care professional with experience in the field, room for subjective categorization still exists. Some questions asked were not aimed for in-depth exploration of the selected areas interest, such that the interpretation of the results should be considered with caution. Nevertheless, these meta-level findings indicate possibly important differences in terms of perceptions and patient management across linguistic regions which deserve further understanding before engaging into large scale programs. Finally, there remains a possibility of unrecognized bias or confounding. As a trade-off between completeness and feasibility, the level of granularity of the physician characteristics was low and the survey did not collect any patient data, which is a limiting factor for more in-depth analyses.
Conclusions
Real-world asthma management and inhaler use in daily practice differ between D-CH and W-CH and with regard to guidelines. Recognizing the specific difficulties related to pMDIs and DPIs, routine inhalation technique monitoring could be considerably improved in both regions. The reasons for higher pMDI preference in W-CH compared to D-CH deserve further research.
Acknowledgements
We are grateful to Dr. Christian Schmidhauser, La Volta Statistics, Meilen Switzerland for supporting the statistical analysis and to Dr Philippe Kress, M.D., Glattbrugg, Switzerland, for his critical review and comments of the manuscript. Study design and statististical analyses were supported by an unrestricted grant of Mundipharma Medical Medical Company, Basel, Switzerland. The sponsor had no influence on the interpretation of the results and the contents of the manuscript.
Footnote
Conflicts of Interest: CF Clarenbach has received payments for lectures/speaking, development of educational materials, and travel/accommodation/meeting expenses from Almirall, AstraZeneca, Boehringer Ingelheim, Fluentis AG, GlaxoSmithKline, Mundipharma, Novartis, Roche AG and Vifor Pharma. And other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the cantonal ethical commission of Zurich, Switzerland (No. 77-2015) and written informed consent was obtained from all patients.
The Asthma Survey Questionnaire
Patient education
1. Which of these practical aspects do you consider when prescribing an inhaled anti-asthmatic drug?
- ⬚ I train with my patient in my office;
- ⬚ My practice assistant trains the patient;
- ⬚ I write a note on the prescription (training by the pharmacist);
- ⬚ I hand out a flyer;
- ⬚ I indicate a link to a patient education video on the Internet;
- ⬚ I recommend reading the package insert;
- ⬚ Other: ______;
- ⬚ None.
2. Which supportive tools for inhalation technique training are missing in daily practice?
_____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________.
3. At which time intervals do you verify the technical appropriateness of your patients’ inhalation technique?
- ⬚ At the next consultation (time interval in weeks_____);
Thereafter every (time interval in weeks________); - ⬚ Only in cases of insufficient asthma control;
- ⬚ Never.
Common errors when using inhalation devices
5. In patients using dry-powder inhalers (DPI), which are the most common errors encountered in daily practice?
6. In patients using pressurized metered-dose inhalers (pMDI), which are the most common errors encountered in daily practice?
Physician preferences & behaviors
7. How many patients with an obstructive airway disease do you treat per week?
- Number of patients with asthma: _________;
- Number of patients with chronic obstructive pulmonary disease (COPD): _________;
- Number of patients with a mixed form: _________________.
8. How many patients with an obstructive airway disease do you treat with a pMDI and how many with a DPI
- ______ % pMDI;
- ______ % DPI.
9. What are the main reasons for prescribing a pMDI?
_____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________.
10. What prior therapy was used in patients newly prescribed a fixed combination of an inhaled corticosteroid (ICS) and a long-acting beta agonist (LABA)?
- ⬚ A short-acting beta-agonist (SABA) only;
- ⬚ A leukotriene receptor antagonist (LTRA);
- ⬚ An ICS in monotherapy;
- ⬚ Another ICS+LABA combination;
- ⬚ Other: ____________.
Note: questions 4 and 11 were launch product related and not relevant to the general survey results.
References
- Leuenberger P, Künzli N, Ackermann-Liebrich U, et al. Swiss Study on Air Pollution and Lung Diseases in Adults (SAPALDIA). Schweiz Med Wochenschr 1998;128:150-61. [PubMed]
- Global Initiative for Asthma (GINA). Poket guide for asthma management and prevention. Available online: www.ginasthma.org, accessed Feb 24, 2016.
- Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J 2002;19:246-51. [Crossref] [PubMed]
- Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: a report from the Aerosol Drug Management Improvement Team. Respir Med 2006;100:1479-94. [Crossref] [PubMed]
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011;105:930-8. [Crossref] [PubMed]
- Basheti IA, Reddel HK, Armour CL, et al. Improved asthma outcomes with a simple inhaler technique intervention by community pharmacists. J Allergy Clin Immunol 2007;119:1537-8. [Crossref] [PubMed]
- Inhaler Error Steering Committee, Price D, Bosnic-Anticevich S, et al. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med 2013;107:37-46. [Crossref] [PubMed]
- Giraud V, Allaert FA, Roche N. Inhaler technique and asthma: feasability and acceptability of training by pharmacists. Respir Med 2011;105:1815-22. [Crossref] [PubMed]
- Bosnic-Anticevich SZ, Sinha H, So S, et al. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J Asthma 2010;47:251-6. [Crossref] [PubMed]
- Kim SH, Kwak HJ, Kim TB, et al. Inappropriate techniques used by internal medicine residents with three kinds of inhalers (a metered dose inhaler, Diskus, and Turbuhaler): changes after a single teaching session. J Asthma 2009;46:944-50. [Crossref] [PubMed]
- Papi A, Haughney J, Virchow JC, et al. Inhaler devices for asthma: a call for action in a neglected field. Eur Respir J 2011;37:982-5. [Crossref] [PubMed]
- Haughney J, Price D, Barnes NC, et al. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med 2010;104:1237-45. [Crossref] [PubMed]
- Lavorini F, Fontana GA, Usmani OS. New inhaler devices - the good, the bad and the ugly. Respiration 2014;88:3-15. [Crossref] [PubMed]
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet 2011;377:1032-45. [Crossref] [PubMed]
- Sadowski CA, Cor K, Cave A, et al. Administration Technique and Acceptance of Inhaler Devices in Patients With Asthma or COPD. Ann Pharmacother 2015;49:639-48. [Crossref] [PubMed]
- Leuppi JD, Steurer-Stey C, Peter M, et al. Asthma control in Switzerland: a general practitioner based survey. Curr Med Res Opin 2006;22:2159-66. [Crossref] [PubMed]
- Steurer-Stey C, Fletcher M, Vetter W, et al. Patient education in asthma: a survey of physicians' knowledge of the principles and implementation of self management in practice. Swiss Med Wkly 2006;136:561-5. [PubMed]
- Burton AJ. Asthma inhalation devices: what do we know? Br Med J (Clin Res Ed) 1984;288:1650-1. [Crossref] [PubMed]
- Interiano B, Guntupalli KK. Metered-dose inhalers. Do health care providers know what to teach? Arch Intern Med 1993;153:81-5. [Crossref] [PubMed]
- Sanchis J, Corrigan C, Levy ML, et al. Inhaler devices - from theory to practice. Respir Med 2013;107:495-502. [Crossref] [PubMed]
- Broeders ME, Sanchis J, Levy ML, et al. The ADMIT series--issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Prim Care Respir J 2009;18:76-82. [Crossref] [PubMed]
- Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011;37:1308-31. [Crossref] [PubMed]
- Basheti IA, Reddel HK, Armour CL, et al. Counseling about turbuhaler technique: needs assessment and effective strategies for community pharmacists. Respir Care 2005;50:617-23. [PubMed]
- Melani AS, Zanchetta D, Barbato N, et al. Inhalation technique and variables associated with misuse of conventional metered-dose inhalers and newer dry powder inhalers in experienced adults. Ann Allergy Asthma Immunol 2004;93:439-46. [Crossref] [PubMed]
- Erickson SR, Chang A, Johnson CE, et al. Lecture versus Web tutorial for pharmacy students' learning of MDI technique. Ann Pharmacother 2003;37:500-5. [Crossref] [PubMed]
- Toumas M, Basheti IA, Bosnic-Anticevich SZ. Comparison of small-group training with self-directed internet-based training in inhaler techniques. Am J Pharm Educ 2009;73:85. [Crossref] [PubMed]
- Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest 2005;127:335-71. [Crossref] [PubMed]
- Lavorini F, Corrigan CJ, Barnes PJ, et al. Retail sales of inhalation devices in European countries: so much for a global policy. Respir Med 2011;105:1099-103. [Crossref] [PubMed]
- Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res 2012;4:184-91. [Crossref] [PubMed]
- Doyle S, Lloyd A, Williams A, et al. What happens to patients who have their asthma device switched without their consent? Prim Care Respir J 2010;19:131-9. [Crossref] [PubMed]
- Thomas M, Price D, Chrystyn H, et al. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med 2009;9:1. [Crossref] [PubMed]
- Welch MJ, Nelson HS, Shapiro G, et al. Comparison of patient preference and ease of teaching inhaler technique for Pulmicort Turbuhaler versus pressurized metered-dose inhalers. J Aerosol Med 2004;17:129-39. [Crossref] [PubMed]
- Lenney J, Innes JA, Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. EDICI. Respir Med 2000;94:496-500. [Crossref] [PubMed]
- Scichilone N, Benfante A, Bocchino M, et al. Which factors affect the choice of the inhaler in chronic obstructive respiratory diseases? Pulm Pharmacol Ther 2015;31:63-7. [Crossref] [PubMed]
- Chrystyn H. Anatomy and physiology in delivery: can we define our targets? Allergy 1999;54 Suppl 49:82-7. [Crossref] [PubMed]
- Tarsin WY, Pearson SB, Assi KH, et al. Emitted dose estimates from Seretide Diskus and Symbicort Turbuhaler following inhalation by severe asthmatics. Int J Pharm 2006;316:131-7. [Crossref] [PubMed]
- Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med 2015;10:13. [Crossref] [PubMed]
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009;64:728-35. [Crossref] [PubMed]
- Asthma, COPD and Asthma-COPD Overlap Syndrome (ACOS). A joint project of GINA and GOLD. Available online: http://www.ginasthma.org/local/uploads/files/ACOS_2015.pdf, accessed Feb 24, 2016. 2015.
- Louie S, Zeki AA, Schivo M, et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol 2013;6:197-219. [Crossref] [PubMed]
- Gibson PG, McDonald VM. Asthma-COPD overlap 2015: now we are six. Thorax 2015;70:683-91. [Crossref] [PubMed]
- Soriano JB, Davis KJ, Coleman B, et al. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest 2003;124:474-81. [Crossref] [PubMed]
- Andersén H, Lampela P, Nevanlinna A, et al. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J 2013;7:342-6. [Crossref] [PubMed]



