Omental flap for treatment of dead space after left upper lobectomy due to aspergilloma
Introduction
Dead space formation in the thoracic cavity after lung parenchymal resection can occasionally lead to intra-thoracic infection. These issues are further complicated in cases of concomitant infection of the surgical cavity, as such infections are often difficult to treat with systemic antibiotics. Common treatment options for cases of dead space in the thoracic cavity include thoracoplasty, eloesser flap, muscle flap, or omental flap. Here, we report the case of a patient who developed chronic empyema in the left upper lung field after pulmonary resection due to aspergilloma, which was successfully treated with omental flap.
Case presentation
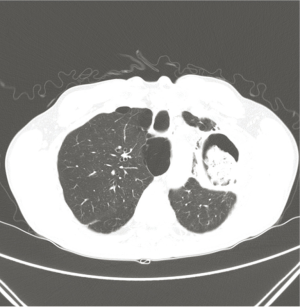
A 55-year-old male patient who had been diagnosed with pulmonary aspergillosis 11 years prior was admitted for treatment due to a recently aggravated hemoptysis. A computed tomography (CT) at the time of admission revealed a cavitary lesion in the left upper lung field containing a fungal ball (Figure 1), indicating the need for resection of the diseased lung.
After general anesthesia, we performed a serratus-sparing posterolateral thoracotomy at the fifth intercostal space. A dense adhesion was detected in the whole lung field, and the major fissure of the left lung was absent, characterized by a consolidation of left upper lobe, which extended to the superior segment of the left lower lobe. Based on these findings, we performed a left upper lobectomy with left lower lobe superior segmentectomy. A postoperative chest X-ray revealed a dead space in the left upper lung field, potentially due to an incomplete expansion of the left lower lobe.
By day 8, the drainage fluid had changed from clear to turbid, identified by in vitro culture as Pseudomonas aeruginosa. The infection was irrigated with betadine solutions for 2 weeks, though the infection was not cleared. We recommended that the patient undergo an eloesser flap or thoracoplasty; however, he refused and was discharged 21 days postoperatively with a Heimlich bag. After discharge, chest CT scans taken in an out-patient clinic showed no significant change in dead space volume in the left upper lung field, and the turbid chest tube drainage persisted. We decided to use an omental flap, rather a muscle flap, in consideration of the patient’s muscle volume, as he exhibited a low body mass index (BMI; 16.8 kg/m2), as well as other sarcopenic features.
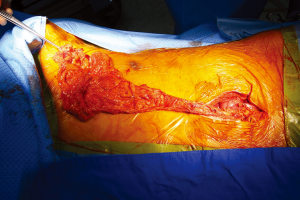
To prepare for the omental flap, we performed an esophagogastroduodenoscopy to screen for occult upper gastrointestinal malignancies, with no significant findings. After general anesthesia, we assessed the volume of the omental tissue using a laparoscope. As the volume of omental tissues was deemed sufficient for a flap, a 7 cm mid-line laparotomy was performed, producing a pedicled omentum (Figure 2). Blunt dissection for formation of a substernal route to the left upper lung field was done, along with massive irrigation with sterile saline. The omental flap was passed through the substernal route and placed in the dead space of the left upper lung field. Several sutures were used to fix the omental flap to the adjacent tissue, and two drainage catheters were placed. After the operation, the dead spaces were shown to be filled with transposed omental flap by both postoperative chest X-ray and chest CT examination (Figure 3).
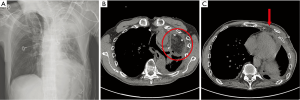
Repeated culture of chest drainage was done, and Pseudomonas aeruginosa was no longer detected, with serum inflammatory markers include C-reactive protein (CRP) level returning to normal. Fever was not developed in postoperative period and CRP was elevated until 3rd postoperative day (23.24 mg/dL), and it was gradually decreased to normal value in 10thpostoperative day. The drainage catheter was completely removed on day 17, and the patient was discharged on day 19 with no signs of infection after 6 months of out-patient follow-up.
Discussion
Insufficient expansion of the residual lung after pulmonary resection is considered a serious complication, particularly in cases of surgically treated infectious pulmonary disease. Thoracoplasty and muscle flap are the most common methods used to treat these dead space problems, with several reports describing successful outcomes in pulmonary aspergilloma patients treated with a combination of cavernostomy, muscle flap, and thoracoplasty (1,2). Here, we considered a muscle flap; however, this procedure was not chosen due to the low BMI of the patient with his sarcopenic features. Other options, such as thoracoplasty or eloesser flap, were not chosen due to marginal pulmonary function, in addition to the patient’s strong objection for cosmetic reasons.
Given the lack of viable options available to treat this patient, we sought to address the dead-space issue using an omental flap. The omental flap has potential for use in a wide range of thoracic surgeries, including chest wall reconstructions (3). A long-term follow-up study examining chronic empyema patients treated with an omental flap also showed encouraging results, with the procedure producing no adverse effects on pulmonary function (4). Under normal circumstances, the omentum serves primarily as a protective tissue in the abdomen, though it has been shown to exhibit a variety of other functions, including cell transport, angiogenesis, absorption of bodily fluids, and immune regulation (5). In patients undergoing the omental flap, this diverse set of functions is thought to aid in the control of local infections, and promote healing in the surrounding tissues. In addition, the amorphous shape and bulk of the omentum offers a significant advantage over a muscle flap due to the greater coverage of dead space (3). In a larger study of the omental flap, the dissected omentum was drawn into the thoracic cavity through an incision in the diaphragm and placed in the target fistula. The omentum was usually transposed to the lower lung fields; however, more than half of all patients required a partial thoracoplasty to completely eliminate the remaining dead space (4).
In case of breast reconstruction after mastectomy, the pedicled omental flap is widely used since 1963; the first reconstructions after mastectomy using pedicled omental flap by Kirikuta (6). In recent, Zaha has reported the favorable outcomes of omental flap for reconstruction after partial mastectomy in upper inner quadrant (7). So we think the length of omental tissues are usually enough to cover the upper thorax, and if the volume of omental tissues is sufficient for flap, the length is not the issue for the procedure.
For this patient, the dissected omentum was drawn through the substernal route without additional injury to the diaphragm. A partial thoracoplasty was not performed, as the omentum was sufficiently mobilized to reach the left apex, enabling coverage of the upper left field.
Preserving as much normal lung tissue as possible is important for avoiding insufficient expansion, particularly in cases of infectious pulmonary disease. We suggest that muscle-sparing incision should be taken into consideration when contemplating lung resection involving more than a segment in suppurative lung disease. And the omental flap may represent a good option for the treatment of chronic empyema, as a result of its immunologic characteristics and greater coverage of dead space, as well as the lower overall morbidity associated with omentectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Grima R, Krassas A, Bagan P, et al. Treatment of complicated pulmonary aspergillomas with cavernostomy and muscle flap: interest of concomitant limited thoracoplasty. Eur J Cardiothorac Surg 2009;36:910-3. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Nagashima T, et al. Pulmonary aspergilloma treated by limited thoracoplasty with simultaneous cavernostomy and muscle transposition flap. Ann Thorac Cardiovasc Surg 2012;18:472-4. [Crossref] [PubMed]
- Shrager JB, Wain JC, Wright CD, et al. Omentum is highly effective in the management of complex cardiothoracic surgical problems. J Thorac Cardiovasc Surg 2003;125:526-32. [Crossref] [PubMed]
- Okumura Y, Takeda S, Asada H, et al. Surgical results for chronic empyema using omental pedicled flap: long-term follow-up study. Ann Thorac Surg 2005;79:1857-61. [Crossref] [PubMed]
- Kitano M.. Omentoplasty in thoracic surgery. Gen Thorac Cardiovasc Surg 2008;56:483-9. [Crossref] [PubMed]
- Claro F Jr, Sarian LO, Pinto-Neto AM. Omentum for Mammary Disorders: A 30-Year Systematic Review. Ann Surg Oncol 2015;22:2540-50. [Crossref] [PubMed]
- Zaha H.. Partial breast reconstruction for the medial quadrants using the omental flap. Ann Surg Oncol 2014;21:3358. [Crossref] [PubMed]


