Thoracoscopic anterior ‘fissure first’ technique for left lung cancer with an incomplete fissure
Introduction
Conventional lobectomy involves separation of branches of the pulmonary artery (PA), pulmonary vein, and bronchus of the corresponding lobe. Exposure of the PA at the fissure is traditionally achieved by dividing the lung parenchyma overlying the artery, usually via electrocautery, blunt dissection, or sharp dissection (1). Dealing with fused fissures can prolong operating time, increase blood loss, and cause morbidities such as air leaks, which can in turn prolong the duration of chest tube drainage and hospitalization (2). For thoracic surgeons, especially during thoracoscopic surgery, dealing with incomplete lung fissures is difficult. There are two approaches to incomplete or fused fissures: retrograde dissection or the fissureless technique (3). Retrograde dissection involves an anterior-to-posterior repair of the interlobar area in a piecemeal fashion. The fissureless technique involves division of the parenchyma as the final step. However, the former cannot be applied to thoracoscopic surgery and the latter may be inappropriate for patients with stage N1 disease in some cases.
We have employed a thoracoscopic anterior ‘fissure first’ technique [also known as ‘fissure first, hilum last’ technique (4)] for dealing with incomplete lung fissures. Here, we describe the details of the technique and the results of our evaluation of its efficacy and safety for left lung cancer with an incomplete fissure.
Methods
Given that individual patients were not identified in this retrospective study, the Ethics Review Board of our institute waived the requirement to obtain written informed consent from patients and approved the study protocol.
Patient groups
One hundred and seventy patients underwent left upper lobectomy or left lower lobectomy in the department of thoracic surgical oncology in our institution between April 2008 and July 2014. Patients in this study were first divided into having unfused or incomplete fissures intraoperatively. Incomplete fissures were defined in cases where, after reasonable dissection (without much damage to the parenchyma in the fissure), we couldn’t identify the PA, and 50% or more of the fissure was fused. Patients who had undergone surgery using a thoracoscopic anterior ‘fissure first’ technique for incomplete lung fissures were allocated to group A, and those treated using a conventional method for unfused fissures were allocated to group B. All surgeons performed both procedures. Air leaks on postoperative day 7, or leaks requiring chemical pleurodesis or reoperation within 7 postoperative days, were defined as ‘prolonged air leaks’. Air leaks that occurred after removal of the chest tube were defined as ‘delayed air leaks’.
Surgical procedure
Patients were placed in the lateral decubitus position under general anaesthesia with single-lung ventilation using a double-lumen endotracheal tube. A four-port complete thoracoscopic approach was used with monitors for the surgeon and the assistant. One 3-cm, one 1.5-cm, and two 7-mm port access incisions were made. Patients in group B underwent a lobectomy with conventional management of fissures using electrocautery for dissecting the fissural parenchyma overlying the PA. Dissection of lung parenchyma overlying the PA was avoided in the group A patients by using the ‘fissure first’ technique.
The thoracoscopic anterior ‘fissure first’ technique consists of the following steps (Figure 1). After incising of the mediastinal pleura at the pulmonary hilum, we first identify the B8 branch of the bronchus located at the cranial aspect of the pulmonary vein and identify the interlobar lymph nodes (position #11). Optimal care is taken with the branches of the PA and the lingular bronchus. The vascular sheath of A8 at the dorsal side of B8 is resected, and the vascular sheath of the PA is detached towards the hilum. Incomplete fissures are then safely divided by placing the anvil of a mechanical stapler (Echelon®, Ethicon, Blue Ash OH, USA) on A8 (Figure 2). A portion of the incomplete fissure is divided towards the posterior aspect using a harmonic scalpel and electrocautery. Finally, a forceps is passed from anterior to posterior after identification of A6 and the fissure division is completed using the stapler. After completion of the fissure, an identical surgical procedure was used for groups A and B. After dissecting the arterial branches of the left upper lobe (LUL) or left lower lobe (LLL), the pulmonary vein was divided. Hilar lymphadenectomy was performed in the conventional manner and the bronchus was divided, thereby completing the lobectomy. For our conventional hilar lymphadenectomy, bronchial arteries were ligated and hilar lymph nodes removed en bloc with the bronchial arteries. In cases of LLL lesions, a subcarinal lymph node dissection was undertaken before dissecting of the LLL bronchus. One of the incisions was extended approximately 3.5 cm to facilitate removal of the resected lobe from the thoracic cavity. A metal chest retractor was not used for this procedure. Fibrin glue (Bolheal®, Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan) and 0.15-mm-thick polyglycolic acid (PGA) mesh (Neoveil®, Gunze Corp., Ltd., Kyoto, Japan) were used as tissue sealants to prevent air leakage.

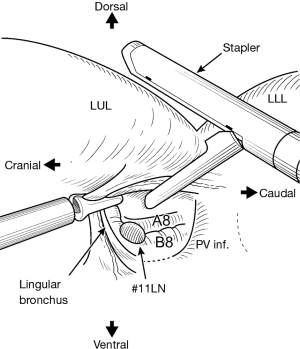
Postoperative care
All patients underwent extubation in the operating room. Thoracic epidural catheters were not inserted in any patients. At the end of the procedure, a water-submersion test was used to detect the presence of air leaks. Distilled water was instilled into the chest cavity and the lung was reinflated to a pressure of 25 cmH2O. In cases where bubbling occurred, every attempt was made to minimize air leakage by applying manual sutures. A single 20-F or 24-F chest tube was placed with suction at a pressure of 5 cmH2O. The next morning, the chest tube was placed on a water seal without suction. Chest radiographs were taken in the operating room immediately after the surgical procedure and on the following morning. Patients were examined every morning and evening until discharge. The decision to remove chest drains was made based on the lack of air leaks with forced expiration, and drainage of <200 mL in the previous 24 h. All patients were discharged after resolution of air leaks, without the use of Heimlich valves.
Data acquisition and follow-up
Preoperative, perioperative, and postoperative details of patients were recorded. Continuous variables are reported as the mean or the mean ± standard deviation and are compared using Student’s t-test. Categorical variables are reported as counts with the corresponding ratios, and are compared using the chi-squared test or Fisher’s exact test. P<0.05 was considered significant. All analyses were conducted using commercial software (SPSS version 20®, IBM, Armonk NY, USA).
Results
Group A consisted of 34 patients and group B of 136 patients. Clinical characteristics of patients are summarized in Table 1. The two groups did not significantly differ with regard to age, sex, smoking history, comorbidity rate, results of lung function tests or preoperative diagnosis. Intraoperative and pathological characteristics are summarized in Table 2. The two groups did not significantly differ with regard to surgical method (P=0.82), extent of lymphadenectomy (P=0.25), operating time (P=0.97), time to lobectomy (P=0.45), blood loss (P=0.73), use of fibrin glue and PGA mesh (P=0.33), duration of chest tube drainage (P=0.49), volume of drainage (P=0.86), or postoperative hospital stay (P=0.28). Only one patient required a staple-line sealant. Patients in group A required more staple cartridges than those in group B (mean, 2.4 vs. 1.1). No patients required conversion to mini-thoracotomy or thoracotomy. Postoperative complications are summarized in Table 2. Prevalence of surgical morbidity was 18% in group A and 14% in group B. In group A, two patients required chemical pleurodesis (OK-432) and one required reoperation. In group B, four patients required chemical pleurodesis while none required reoperation. Although there was no significant difference between left upper lobectomies and lower lobectomies of the two groups, the incidence of air leaks tended to be higher in left upper lobectomies of group A (Table 3). No patients developed bronchopleural fistulae. No patients died. For all patients in group A, hilar lymphadenectomy could be performed in the conventional manner.
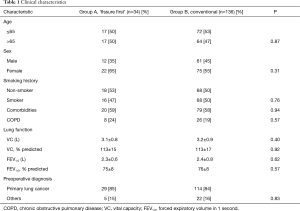
Full table
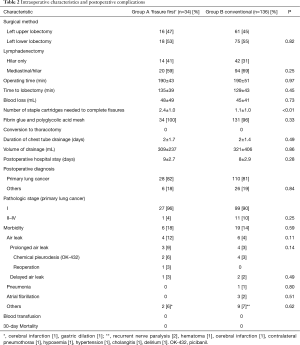
Full table
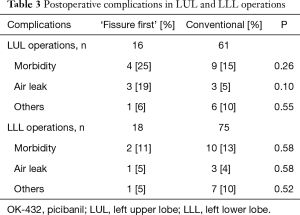
Full table
Discussion
Dealing with incomplete lung fissures during thoracoscopic surgery is difficult. Generally, there are two techniques to approach incomplete or fused fissures (3). One technique for incomplete fissures is retrograde dissection. In this method, the presence of the PA behind the pulmonary hilum is first confirmed. Detachment proceeds along the course of the PA, and the surface of the lung parenchyma is then ligated. This process is repeated, allowing repair of the interlobar area in a piecemeal fashion. However, this method can only be applied to open thoracotomy, as manipulation of forceps via access ports is limited. In contrast, the thoracoscopic anterior ‘fissure first’ technique can be applied to thoracoscopic surgery.
Dissection through the fissure to gain access to the PA can increase the risk of postoperative air leaks, and a ‘fissureless technique’ has been reported in which the final step is usually division of the parenchyma with staplers (6-12). In a randomized trial that included resection of all lobes, Gomez-Caro and colleagues reported that the ‘fissureless technique’ resulted in a significantly lower incidence of prolonged air leaks than the conventional technique and a higher probability of cessation of air leaks without an increase in morbidity and mortality (8). However, the ‘fissureless technique’ may be inappropriate for patients with stage N1 disease due to the risk of damage to lymph nodes. When we perform the ‘fissureless technique’, we will incise between left upper or lower bronchus and lymph nodes (position #12u or #12l) in blind to some extent. We will not view in some cases whole hilar lymph nodes in this technique. On the other hand, the ‘fissure first’ technique enable look at whole lymph nodes during lymphadenectomy by completion of the fissure.
This study is not randomized study. The judgment of an incomplete fissure or not depends on surgeons. One surgeon (MM) is engaged in all cases and has responsibility of all cases. He and his colleague try to perform ‘fissure first’ technique. Group A and B are performed at the same period.
The incidence of prolonged air leaks can be 5–15%, depending on how prolonged air leaks are defined (2,13-15). Our results were not inferior to those of previous reports. There was no significant difference in the prevalence of air leak in patients who underwent the thoracoscopic anterior ‘fissure first’ technique and in those who underwent the conventional procedure. In the ‘fissure first’ group, the incidence of air leaks tended to be higher in LUL operations than LLL operations, although there was no significant difference. The thoracoscopic anterior ‘fissure first’ technique in patients with an incomplete fissure should be further considered for use in the LLL operations.
To avoid air leaks and unexpected bleeding with the thoracoscopic anterior ‘fissure first’ technique, the use of mechanical staplers is preferable when completing fused fissures. The use of these staplers might promote the expansion of the residual lung and benefit postoperative lung function. This makes the thoracoscopic anterior ‘fissure first’ technique more expensive than the conventional method. Given that one staple cartridge costs approximately USD $250 in Japan, the mean cost for patients with incomplete fissures was $325 more than that for patients with unfused fissures. However, as hospital stay was not prolonged, the overall medical cost was not increased. The fissureless technique with an open surgery required 3.2 staple cartridges (8).
In the present study, haemorrhage was not observed in any patients with fused fissures. The thoracoscopic anterior ‘fissure first’ technique requires a high degree of anatomical knowledge to prevent haemorrhage. Preoperative computed tomography (CT) imaging is employed to assess for three important variables: the presence of a common trunk of the pulmonary vein, the presence of the lingular PA branching from A8, and the distance between A1+2c and A6. The thoracoscopic anterior ‘fissure first’ technique is more difficult when the pulmonary vein has a common trunk and the lingular PA branches from A8. Further, when the distance between the branch of A1+2 and A6 is short, care must be taken when the forceps is passed from anterior to posterior. Although four patients had these three findings, the thoracoscopic anterior ‘fissure first’ technique could be conducted in all of them. Detailed preoperative CT studies are therefore essential.
The present study is limited by its small sample size and observational retrospective design. Although propensity score matching is effective, quantifying the degree of lung lobulation is difficult. Confirmation of our results requires a prospective, randomized, controlled clinical trial.
We found that the thoracoscopic anterior ‘fissure first’ technique for left lung cancer with an incomplete fissure enabled hilar lymphadenectomy to be performed in the conventional manner without any increase in the prevalence of air leaks, operating time, or duration of chest tube drainage. The thoracoscopic anterior ‘fissure first’ technique for an incomplete fissure should therefore be considered for left lung cancer, especially LLL cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Given that individual patients were not identified in this retrospective study, the Ethics Review Board of our institute waived the requirement to obtain written informed consent from patients and approved the study protocol.
References
- Temes RT, Willms CD, Endara SA, et al. Fissureless lobectomy. Ann Thorac Surg 1998;65:282-4. [Crossref] [PubMed]
- Stolz AJ, Schützner J, Lischke R, et al. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:334-6. [Crossref] [PubMed]
- Shields TW. General features of pulmonary resections. In: Shields TW, Locicero J 3rd, Reed CE, et al. editors. General Thoracic Surgery. 7th ed. Philadelphia: Lippincott Williams and Wilkins, 2009:401-12.
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Samejima J, Mun M, Matsuura Y, et al. After incising of the mediastinal pleura at the pulmonary hilum, we first identified the B8 branch of the bronchus located at the cranial aspect of the pulmonary vein and confirmed interlobar lymph nodes (position #11). Asvide 2016;3:462. Available online: http://www.asvide.com/articles/1236
- Yim AP, Izzat MB, Liu HP, et al. Thoracoscopic major lung resections: an Asian perspective. Semin Thorac Cardiovasc Surg 1998;10:326-31. [Crossref] [PubMed]
- Nomori H, Ohtsuka T, Horio H, et al. Thoracoscopic lobectomy for lung cancer with a largely fused fissure. Chest 2003;123:619-22. [Crossref] [PubMed]
- Gómez-Caro A, Calvo MJ, Lanzas JT, et al. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardiothorac Surg 2007;31:203-8. [Crossref] [PubMed]
- Ng T, Ryder BA, Machan JT, et al. Decreasing the incidence of prolonged air leak after right upper lobectomy with the anterior fissureless technique. J Thorac Cardiovasc Surg 2010;139:1007-11. [Crossref] [PubMed]
- Balsara KR, Balderson SS, D'Amico TA. Surgical techniques to avoid parenchymal injury during lung resection (fissureless lobectomy). Thorac Surg Clin 2010;20:365-9. [Crossref] [PubMed]
- Refai M, Brunelli A, Salati M, et al. Efficacy of anterior fissureless technique for right upper lobectomies: a case-matched analysis. Eur J Cardiothorac Surg 2011;39:1043-6. [Crossref] [PubMed]
- Carrott PW Jr, Jones DR. Teaching video-assisted thoracic surgery (VATS) lobectomy. J Thorac Dis 2013;5 Suppl 3:S207-11. [PubMed]
- Venuta F, Rendina EA, De Giacomo T, et al. Technique to reduce air leaks after pulmonary lobectomy. Eur J Cardiothorac Surg 1998;13:361-4. [Crossref] [PubMed]
- Brunelli A, Monteverde M, Borri A, et al. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg 2004;77:1205-10; discussion 1210. [Crossref] [PubMed]
- Rice TW, Okereke IC, Blackstone EH. Persistent air-leak following pulmonary resection. Chest Surg Clin N Am 2002;12:529-39. [Crossref] [PubMed]


