Comparison of outcomes of tricuspid annuloplasty with 3D-rigid versus flexible prosthetic ring for functional tricuspid regurgitation secondary to rheumatic mitral valve disease
Introduction
Functional tricuspid regurgitation (FTR) is common in patients with rheumatic mitral valve disease (1,2). The primary causes of FTR are annular dilation and right ventricular enlargement which may lead to tricuspid anatomical and functional abnormalities (3). FTR is often secondary to left heart failure from myocardial or valvular causes, right ventricular volume and pressure overload, and dilation of cardiac chambers. Secondary (functional) tricuspid regurgitation (TR) is associated with poor outcome and predicts poor survival, heart failure, and reduced functional capacity (4). However, the optimal surgical technique such as repair vs. replacement, access, type of prosthesis to rectify TR remains challenging (5).
Due to the high-risk of postoperative complications after tricuspid valve replacement, repair is currently the preferred surgical method for FTR (6). The two principal surgical methods of tricuspid valve repair include suture and prosthetic annuloplasty (i.e., flexible band and remodeling ring). Suture annuloplasty, such as the Kay method (7) and the De Vega method (8), are routinely used for tricuspid valve repair by bicuspidization or reduce annular size semicircularly. It has the advantages of technically easy and low patient’s economic burden, however associated with relatively high recurrent TR rate (9,10). Ring annuloplasty can reduce or remodel the dilated annulus by using prosthetic rings (flexible or rigid). Compared with suture methods, prosthetic ring annuloplasty may be associated with better prevention of annular dilation, right ventricular volume overload and right ventricular failure (11). Ring annuloplasty is currently favored for surgical treatment of secondary TR. However, despite the advantages of ring annuloplasty for functional TR (5), the efficacy and durability of specifically shaped tricuspid prosthetic rings (i.e., flexible or rigid) have not yet been thoroughly elucidated.
Currently, whether flexible or rigid rings should be used remains controversial. Navia et al. (12) and Izutani et al. (13) reported a lower rate of recurrent TR with a rigid annuloplasty ring compared with a flexible ring. On the contrast, Pfannmüller et al. (14) reported a higher rate of annuloplasty dehiscence after the implantation of a rigid ring. In the present study, the purpose was to compare the efficacy and mid-term durability of tricuspid ring annuloplasty for FTR secondary to rheumatic mitral valve disease using Cosgrove-Edwards flexible band and the Edwards MC3 rigid ring (Edwards Lifesciences, LLC, Irvine, CA, USA).
Methods
This retrospective study was approved by the ethics committee of Qingdao Fuwai Hospital (No. 002/2009) and conducted in accordance with the Declaration of Helsinki. Written informed consent before the surgical procedures and for the use of personal information for research purposes was obtained from each patient. We retrospectively collected and analyzed the clinical data of patients undergoing mitral valve replacement (MVR) and tricuspid ring annuloplasty using Cosgrove-Edwards flexible band or Edwards MC3 rigid ring for FTR with concomitant rheumatic mitral valve disease at our hospital from September 2009 to December 2013. The indications for tricuspid ring annuloplasty were moderate and above FTR. Patients with tricuspid insufficiency caused by congenital tricuspid valve abnormalities or primary lesion such as trauma, infective endocarditis and autoimmune disease were excluded from this study. Patients who were treated with simultaneous aortic valve replacement, coronary artery bypass surgery, radiofrequency ablation of atrial fibrillation, and surgical correction of congenital heart disease were also excluded.
Preoperative echocardiographic assessment
All patients were assessed preoperatively by transthoracic two-dimensional and color Doppler echocardiography. The severity of TR were evaluated using the apical four chamber view and was graded as 0 for no regurgitation, 1+ for mild regurgitation, 2+ for moderate regurgitation, 3+ for moderately severe regurgitation, and 4+ for severe regurgitation (5,15).
Surgical procedures
All surgeries were performed through median sternotomies with bicaval and aortic cannulation and standard hypothermic cardiopulmonary bypass (CPB) by the same surgeon. After clamping the ascending aorta, the right atrium and interatrial septum were dissected. If there existed left atrial thrombus, thorough clearage can be achieved bluntly or vacuum aspiration as appropriate. Thereafter, tricuspid valve repair was performed with flexible band or with rigid ring annuloplasty following concomitant MVR and closure of atrial septal incision. In this study, two kinds of tricuspid annuloplasty devices were used: Cosgrove-Edwards flexible band and Edwards MC3 rigid ring. For patients receiving flexible band, tricuspid ring annuloplasty was done with the use of the Cosgrove-Edwards annuloplasty system. Seven to ten 2–0 Ethibond Excel sutures (Ethicon Endo-Surgery, LLC, USA) were placed on the annulus along the anterior and posterior leaflets. A Cosgrove-Edwards flexible band was tied down with the sutures and placed on the annulus under cardiac arrest. For patients receiving rigid ring, tricuspid ring annuloplasty were done with the use of the Edwards MC3 tricuspid annuloplasty system. Nine to eleven 2–0 Ethibond Excel sutures were placed on the annulus, running from the anteroseptal commissure to the middle of the septal leaflet along the anterior and posterior leaflets. A MC3 rigid ring was tied down with the sutures and placed on the annulus under cardiac arrest. In both conditions, the ring size was determined by the length between the commissures along the septal leaflet under cardiac arrest. The prosthetic valves used during MVR were all On-X Mitral Prothetic Heart Valve (On-X Life Technologies, Inc., USA).
Follow-up
After surgery, patients were followed up on postoperative day seven and regularly every three to six months. The patients were followed at outpatient clinic in our hospital. During the follow up period, patient’s clinical status and echocardiographic results were obtained by the cardiologists.
Statistical analysis
All data analyses were performed by using the Statistical Package, version 17.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as numbers and/or percentage and compared using the χ2 test or Fisher’s exact test as appropriate. Continuous variables are expressed as mean ± standard deviation (SD) and compared by Student’s t-test or Mann- Whitney U test. Survival probabilities were constructed using Kaplan–Meier survival estimates. Comparisons between survival curves were performed by using the log-rank test. The differences were considered statistically significant at a P<0.05.
Results
Baseline data
A total of 106 patients undergoing MVR and tricuspid ring annuloplasty for FTR with concomitant rheumatic mitral valve disease were included in this study. The flexible bands were used in 46 patients (flexible group) between September 2009 and November 2011. Since then, the rigid rings were used in the remaining 60 cases (rigid group) between December 2011 and December 2013. The mean age of patients in flexible group and rigid group was 56±5.2 years old and 58±4.8 years old, respectively. The degrees of preoperative FTR were 3.31±0.39 and 3.47±0.31 in flexible group and rigid group, respectively. There was no significant difference between the two groups regarding age, sex, BMI, New York Heart Association (NYHA) functional classification, Mitral disease, degree of FTR, tricuspid annular diameter, tethering distances, right ventricular diameter (RVD), left ventricular ejection fraction (LVEF), and left ventricular end diastolic dimension (LVDD) (all P>0.05) (Table 1).
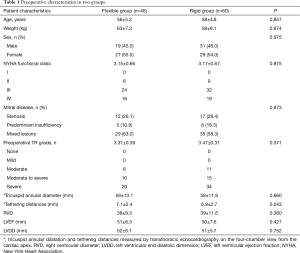
Full table
Operative characteristics and Mortality
Operative outcomes in two groups are shown in Table 2. There was no statistical significant difference regarding prothetic mitral valve size, annuloplasty ring size, operation time, CPB time, cardiac arrest time, intensive care unit (ICU) stays, hospital stays, and ventilator time mortality between the flexible group and the rigid group (all P>0.05).
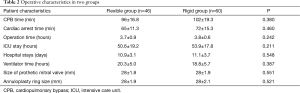
Full table
Mortality and follow up
Postoperatively, three were two cases (including 1 of 46 cases in flexible group and 1 of 60 cases in rigid group) who died during hospitalization, giving a hospital mortality rate of 1.9%. Late death occurred in five patients including two cases in flexible group and three cases in rigid group. The overall mortality was 3 of 46 (6.5%) in flexible group and 4 of 60 (6.7%) in rigid group (P>0.05). The causes of hospital and late mortality were shown in Table 3.
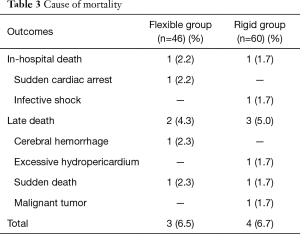
Full table
The mean follow-up was 34.5±9.3 months (range, 24–48 months). Follow up of 93 cases (90%) was successfully achieved. During the follow-up period, there was one case of infective endocarditis in each group, who occurred at 8 months after surgery (flexible group) and 2 years after surgery (rigid group), respectively. Between 30 and 40 months after surgery, echocardiography was performed in 15 cases in flexible group, and 22 cases in rigid group. There were no prosthetic-related complications such ring dehiscence, thromboembolic event in two groups during the follow up period.
Cardiac function improvement was seen in all surviving patients after operation. The average NYHA functional class was significantly improved compared to preoperative values in two groups (all P<0.01) (Table 4). There was no significant difference regarding postoperative NYHA functional class between the two groups at postoperative day 7, 2–3 months, and 6–12 months but there was statistical significant difference at 2–3 years. These may be due to three-dimensional MC3 rigid ring is able to provide better long-term stability of tricuspid valve repair.
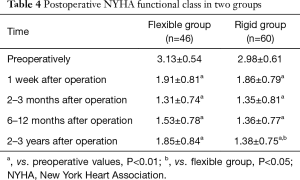
Full table
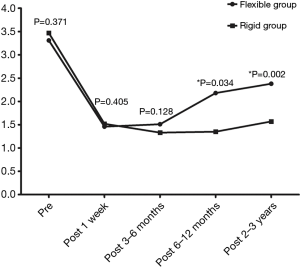
During the follow up period, the grade of TR was greatly improved compared to preoperative values for patients in both flexible and rigid groups (all P<0.05) (Table 5). Changes of preoperative and postoperative TR grade in flexible group and rigid group were shown in Figure 1. There was no significant difference regarding postoperative TR grade between the two groups at 1 week and 2–3 months but there was statistical significant difference at postoperative 6–12 months, and 2–3 years.
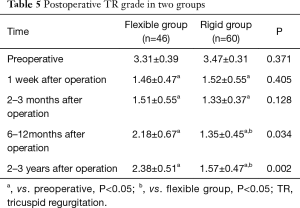
Full table
Recurrent TR was defined as postoperative moderate and above TR (grade 2–4). During the follow up period, 25 of 46 patients (54.3%) in flexible group and 22 of 60 patients (30.3%) in rigid group developed recurrent TR. Freedom from recurrent TR in two groups was shown in Figure 2. Freedom from recurrent TR in flexible group is lower than rigid group in each postoperative follow up period (P=0.046). 28 or 30 mm annuloplasty ring use, LVDD >55 mm, NYHA functional class III–IV, tethering distances >8 mm RV dimension >40 or MAZE procedure was not a predictor for recurrent TR identified by univariate analysis of the patients with recurrent TR (Table 6).
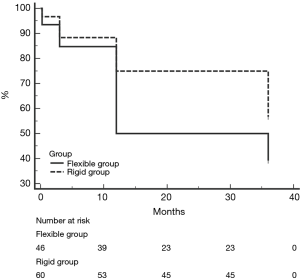
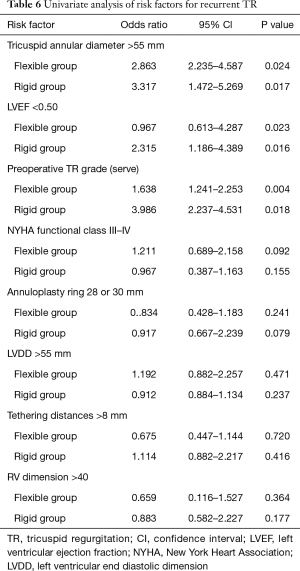
Full table
Discussion
TR is one of the most commonly encountered valvular problems in clinical practice. More than 80% of TR encountered in clinical practice is secondary or functional in nature. FTR usually results from left-sided heart disease, most often MV stenosis or regurgitation (16). The common mechanism of FTR is annular dilatation and leaflet tethering. The principles of surgery for FTR include elimination of increased right ventricular afterload (e.g., by correction of left-sided valve disease) and correction of tricuspid annular dilatation. Currently, the best method of correcting FTR in terms of timing and surgical techniques is still debated. Recently, valve annuloplasty is the preferred surgical treatment for FTR (17-19). However, to date there is no clear evidence of the superiority of one annuloplasty device over the other (14,19). To our knowledge, this is the first report explored and compared the efficacy and mid-term outcomes of the tricuspid ring annuloplasty for FTR secondary to rheumatic mitral valve disease using flexible band and rigid ring. In this study, we found that rigid ring annuloplasty was inclined to be better in restoring and maintaining tricuspid valve function in mid-term postoperative periods.
Pfannmüller et al. (14) investigated a large number of patients with either a flexible band and found that use of a rigid ring increases risk of subsequent ring dehiscence. In addition, Galiñanes et al. (20) and Kay et al. (21) reported four cases of extremely rare complication, i.e., fracture of the Carpentier rigid ring in the tricuspid position. In this study, we observed no occurrence of ring dehiscence in rigid group during the postoperative and follow period. These may be due to be utilized one suture spanning the conjunction region of valve leaflet rather than two sutures on each side of the valve leaflet.
In this study, the hospital mortality and late death rate were similar in the two groups, but was lower that of the previous literatures. These may be due to the high homogeneity of our samples. With regard to improvement of cardiac function, we found significant improvement when compared to preoperatively. Our results were consistent with the previous studies (22,23). In addition, we found no significant difference between the two groups at the postoperative early periods (within 1 year) but significant difference 2 years after surgery. Our study demonstrated that 3D rigid ring annuloplasty had more efficacies in restoring and maintaining tricuspid valve function in early and mid-term postoperative periods.
McCarthy et al. (5) reported that grade of TR was stable across time with the rigid ring and increased slowly with the flexible band. TR grade at discharge and the follow-up period showed better results in the rigid group. Navia et al. (12) compared two large groups (rigid ring: 584; flexible band: 1,052) of using rigid ring and flexible band for TR secondary to left-sided valve disease and found that patients with either standard or 3D rigid prosthetic ring annuloplasty had the least increase across time compared with those receiving flexible rings after 5-year of follow-up. In this study, we found that the grade of TR after surgery and at the follow up period was greatly improved compared to preoperative values for patients in both of flexible and rigid groups. Furthermore, we found that the grade of TR was relative stable across time with the rigid ring and increased slowly with the flexible band. Moreover, we found that the postoperative grade of TR for patients receiving rigid rings was relatively lower compared to that of patients receiving flexible bands at 2–3 years of follow up. This may be due to rigid MC3 annuloplasty ring has a 3-dimensional design and is preconfigured to accommodate the saddle shape of the annulus. In contrast, a flexible band follows physiological motion of the tricuspid annulus during cardiac cycle, but may not keep the optimal saddle shape of the tricuspid annulus. Our results were consistent with the above-mentioned literatures.
Filsoufi et al. (22) suggested that systematic downsizing of the prosthetic ring instead of type of the ring had probably played an important role in reducing the incidence of significant residual or recurrent TR, or both, after ring annuloplasty. On the contrast, McCarthy et al. (5) reported that the size of the ring was not identified as a risk factor for recurrent TR. They concluded that the strategy of undersizing the tricuspid annulus for functional TR using small rings could not be validated as protection against late recurrent TR. In this study, there is no difference of prosthetic ring size in two groups. In addition, univariate analysis showed that large rings (28 or 30 mm) use was not a risk factor for recurrent TR in both flexible and rigid ring groups. Therefore, ring size was not a risk factor in this study.
In this study, annuloplasty with rigid ring provided better result regarding postoperative incidence of recurrent TR, NYHA functional class, grade of TR and freedom of recurrent TR than those with flexible ring for FTR secondary to rheumatic mitral valve disease in mid-term outcome. These findings suggest the superiority of rigid ring. However, it should be noted that there was also a major difference in the technique of implantation. The flexible band was implanted from the anteroseptal to posteroseptal commissure, whereas the rigid ring was implanted from the anteroseptal commissure to the middle of the septal leaflet. McCarthy and Cosgrove recommended the use of Cosgrove band (24). Whereas Filsoufi et al. (22) recommended the use of Edwards MC3 remodeling ring due it is easy to implant and significantly reduces functional TR. The flexible Cosgrove-Edwards band follows physiological motion of the tricuspid annulus during cardiac cycle, but may not keep the optimal saddle shape of the tricuspid annulus. On the contrary, the Edwards MC3 annuloplasty system has a 3-dimensional design and is preconfigured to accommodate the saddle shape of the annulus. Therefore, implantation of the band from the anteroposterior to the posteroseptal commissures—but not up to the middle of the septal leaflet – could potentially compromise the anchoring of the Cosgrove band, which could possibly lead to increased chances of recurrent TR. However, it will be interesting to achieve more value information if new flexible band which can be implanted form the anteroposterior to the middle of the septal leaflet can be developed in the future. It should also be noted that the incidence of recurrent TR at 2 years after annuloplasty with rigid ring still reach to as high as 30.3%. These may be associated with the surgical timing due to patients in China are inclined to receive surgical treatment only when the cardiac function was seriously impaired. Therefore, more aggressive efforts should still be paid to improve the long term outcomes of annuloplasty.
The limitations of this study include its single-center design and retrospective nature, with all of the inherent limitations of such investigations. A non-randomized study with relatively short duration of follow up and small sample size may produce potential bias. Therefore, the results obtained can in no way be considered conclusive and should be confirmed by further studies. Nevertheless, our findings may help cardiac surgeons select an appropriate annuloplasty ring technique for the treatment of TR secondary to rheumatic heart valve disease.
Conclusions
Both flexible and rigid ring annuloplasty have been proved to be safe, feasible and durable to correct secondary TR. The three-dimensional MC3 rigid ring is inclined to be better than the flexible band in terms of postoperative NYHA functional class, grade of TR and recurrent TR in early and mid-term postoperative periods. However, further studies with a larger number of samples and a longer-term of follow up are necessary to confirm our findings. In addition, the potential effect of the two different techniques of ring implantation on the efficacy and outcome of treatment for secondary TR should also be further investigated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of Qingdao Fuwai Hospital (No. 002/2009) and written informed consent was obtained from all patients.
References
- Li ZX, Guo ZP, Liu XC, et al. Surgical treatment of tricuspid regurgitation after mitral valve surgery: a retrospective study in China. J Cardiothorac Surg 2012;7:30. [Crossref] [PubMed]
- Antunes MJ, Barlow JB. Management of tricuspid valve regurgitation. Heart 2007;93:271-6. [Crossref] [PubMed]
- Dreyfus GD, Martin RP, Chan KM, et al. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol 2015;65:2331-6. [Crossref] [PubMed]
- Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol 2009;53:401-8. [Crossref] [PubMed]
- McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 2004;127:674-85. [Crossref] [PubMed]
- Arsalan M, Walther T, Smith RL 2nd, et al. Tricuspid regurgitation diagnosis and treatment. European Heart Journal 2015. [Crossref] [PubMed]
- Kay JH, Maselli-Campagna G, Tsuji KK. Surgical Treatment of Tricuspid Insufficiency. Ann Surg 1965;162:53-8. [Crossref] [PubMed]
- De Vega NG. Selective, adjustable and permanent annuloplasty. An original technic for the treatment of tricuspid insufficiency. Rev Esp Cardiol 1972;25:555-6. [PubMed]
- Ren WJ, Zhang BG, Liu JS, et al. Outcomes of tricuspid annuloplasty with and without prosthetic rings: a retrospective follow-up study. J Cardiothorac Surg 2015;10:81. [Crossref] [PubMed]
- Bernal JM, Pontón A, Diaz B, et al. Combined mitral and tricuspid valve repair in rheumatic valve disease: fewer reoperations with prosthetic ring annuloplasty. Circulation 2010;121:1934-40. [Crossref] [PubMed]
- Tang GH, David TE, Singh SK, et al. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation 2006;114:I577-81. [Crossref] [PubMed]
- Navia JL, Nowicki ER, Blackstone EH, et al. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg 2010;139:1473-1482.e5. [Crossref] [PubMed]
- Izutani H, Nakamura T, Kawachi K. Flexible band versus rigid ring annuloplasty for functional tricuspid regurgitation. Heart Int 2010;5:e13. [Crossref] [PubMed]
- Pfannmüller B, Doenst T, Eberhardt K, et al. Increased risk of dehiscence after tricuspid valve repair with rigid annuloplasty rings. J Thorac Cardiovasc Surg 2012;143:1050-5. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Shinn SH, Dayan V, Schaff HV, et al. Outcomes of ring versus suture annuloplasty for tricuspid valve repair in patients undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2016;152:406-415.e3. [Crossref] [PubMed]
- Huang X, Gu C, Men X, et al. Repair of functional tricuspid regurgitation: comparison between suture annuloplasty and rings annuloplasty. Ann Thorac Surg 2014;97:1286-92. [Crossref] [PubMed]
- Parolari A, Barili F, Pilozzi A, et al. Ring or suture annuloplasty for tricuspid regurgitation? A meta-analysis review. Ann Thorac Surg 2014;98:2255-63. [Crossref] [PubMed]
- Zhu TY, Wang JG, Meng X. Is a rigid tricuspid annuloplasty ring superior to a flexible band when correcting secondary tricuspid regurgitation? Interact Cardiovasc Thorac Surg 2013;17:1009-14. [Crossref] [PubMed]
- Galiñanes M, Duarte J, de Caleya DF, et al. Fracture of the Carpentier-Edwards ring in tricuspid position: a report of three cases. Ann Thorac Surg 1986;42:74-6. [Crossref] [PubMed]
- Kay HR, Hammond GL. Fracture of a prosthetic tricuspid annular ring. J Thorac Cardiovasc Surg 1982;83:635. [PubMed]
- Filsoufi F, Salzberg SP, Coutu M, et al. A three-dimensional ring annuloplasty for the treatment of tricuspid regurgitation. Ann Thorac Surg 2006;81:2273-7. [Crossref] [PubMed]
- Gatti G, Maffei G, Lusa AM, et al. Tricuspid valve repair with the Cosgrove-Edwards annuloplasty system: early clinical and echocardiographic results. Ann Thorac Surg 2001;72:764-7. [Crossref] [PubMed]
- McCarthy JF, Cosgrove DM 3rd. Tricuspid valve repair with the Cosgrove-Edwards Annuloplasty System. Ann Thorac Surg 1997;64:267-8. [Crossref] [PubMed]


