Relationship between survival and age in patients with idiopathic pulmonary fibrosis
Introduction
Idiopathic pulmonary fibrosis (IPF) has an overall poor prognosis. The median survival time of patients with IPF was 2–3 years from the time of diagnosis, in several retrospective longitudinal studies (1-6). There are limited data on clinical factors associated with increased mortality (4). Poor prognostic baseline factors for survival of patients with IPF are the level of dyspnea, diffusing capacity of the lung for carbon monoxide (DLco) <40% predicted, desaturation <88% during the 6-minute walk test, extent of honeycombing on high-resolution computed tomography of the chest, and pulmonary hypertension (4). A simplified scoring system using age, respiratory hospitalization, percent predicted forced vital capacity (FVC), and 24-week change in FVC was developed for determining prognosis and guiding clinical management (7). However, there is no generally accepted method of combining these predictors to determine an accurate prognosis. Whether older patients with IPF have a worse prognosis is controversial. Several studies (4-6,8-10) have suggested that old age may be associated with bad prognosis in IPF.
Observed and relative survival are two main methods of quantifying patient survival (11). Relative survival rate is the ratio of observed survival rate to expected survival rate. Expected survival rate is the survival rate in a population without the disease. The analysis of relative survival rate is generally used in population-based cancer registries (11-13). Relative survival analytic methods are often used to analyze aging-related disease to overcome the potential bias associated with age (14). The merit of the relative survival model is that it adjusts for the disease-associated mortality associated with age separately from expected mortality in the general population.
Relative survival rate has not been used widely for IPF. The Korean Interstitial Lung Disease (ILD) Research Group carried out a national, multicenter survey to evaluate the clinical, physiological, and radiological aspects of IPF [Korean national survey of idiopathic interstitial pneumonia (IIP)] (15,16). The aim of this study was to assess whether age can affect survival in patients with IPF and to assess the difference between observed and relative survival rated according to age in a large population using Korean national survey data.
Methods
This study was a large retrospective multicenter study. The Korean ILD Research Group, comprising 54 universities and training hospitals, enrolled patients newly diagnosed with IPF by pulmonology specialists (national survey of IIP in Korea). Patients were diagnosed with IIP from January 1, 2003 to December 31, 2007. The patients’ medical records were entered into the Korean ILD web-based registry (www.ild.or.kr). We collected the data using a web-based registry from February 2008 to July 2008.
The Scientific Committee of the Korean Academy of Tuberculosis and Respiratory Diseases reviewed all enrolled patients. Patients with clinical evidence of underlying connective tissue disease or any other cause of interstitial disease were excluded from the study. In total, 2,186 patients with IIP were registered to the Korean IIP national survey.
We re-analyzed the Korean national survey data, consisting of 1,685 patients with IPF by clinical diagnosis (1,027 patients) or pathological diagnosis (658 patients) according to the 2002 ATS/ERS criteria (6). Twenty-two cases with incomplete data related to their smoking history were excluded. Consequently, 1,663 medical records of patients with IPF were evaluated including treatment, date of death or final follow-up, and cause of death in this study. We assessed the overall survival rate of patients with IPF. We divided by treatment the group of patients treated with corticosteroids or cytotoxic therapy during follow-up and only conservative treatment.
We divided 1,663 patients into three age groups (<60, 60–69, and ≥70 years).
The baseline clinical characteristics included sex, smoking history, comorbidities [hypertension, diabetes mellitus (DM), heart disease, tuberculosis, chronic obstructive pulmonary disease (COPD), lung cancer], percentage of predicted forced vital capacity [(FVC) (% pred)], percentage of predicted forced expiratory volume in 1 second [FEV1 (% pred)], percentage of predicted total lung capacity [TLC (% pred)], DLco (% pred), arterial oxygen pressure (PaO2), and arterial pressure of carbon dioxide (PaCO2) at initial diagnosis.
Statistical analysis
Differences in baseline clinical characteristic among three age groups were assessed using Pearson chi-square test or analysis of variance, as appropriate.
Survival distributions were computed using the Kaplan-Meier method and differences in survival among the groups, with factors of interest, were compared using the log-rank test. The Cox proportional hazards model was used to determine the effect of various factors on survival.
Relative survival rate in patients with IPF was the ratio of observed survival rate in patients with IPF to expected survival rate in the general Korean population within the same calendar year, sex, and age, and was computed using the 2003–2008 Korean Census life tables of sex and age-specific mortality in open sources from the Korean Statistical Information Service (http://kosis.kr/).
Multivariate survival analyses were performed using the relative survival model with proportional excess hazard and Poisson regression. All statistical analyses were conducted using STATA software (version 11.0; StataCorp, College Station, TX, USA).
Ethics statement
This study protocol was reviewed and approved by the Institutional Review Board (IRB) of each hospital, and exempted from approval by the IRB of Soonchunhyang University Seoul Hospital (IRB file number: SCHUH 2014-09-007), which deemed that informed consent was unnecessary.
Results
Baseline clinical characteristics
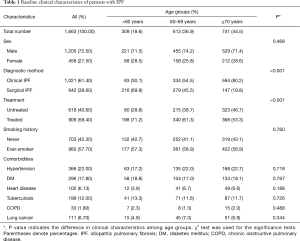
Among the 1,663 patients with IPF, mean age was 67.9 years (range, 30–94 years). The three age groups were: <60 years, n=309; 60–69 years, n=613; and ≥70 years, n=741. Baseline clinical characteristics are shown in Table 1. There were 458 (27.5%) women and 1,205 (72.5%) men. About 39% of patients with IPF were diagnosed by surgical lung biopsy. About 39% of patients with IPF were diagnosed by surgical lung biopsy. The majority (59.4%) of patients were treated with corticosteroids or cytotoxic therapy. Never-smokers accounted for 42.3% (703/1,663) of patients. The most common comorbidity was hypertension.
Significant differences were observed in the diagnostic method among age groups (P<0.001) (Table 1). The older age groups tended to have a higher number of clinical diagnoses. There was a difference between the treatment groups in regard to the proportions according to age (P<0.001). The proportion of receiving treatment with corticosteroids or cytotoxic therapy was higher in the group of younger patients. The proportion of never smokers was not different between the age groups. No differences in comorbidities (hypertension, DM, heart disease, tuberculosis, COPD, and lung cancer) were observed among age groups.
Full table
Pulmonary function and laboratory data at initial diagnosis
Pulmonary function and laboratory data at initial diagnosis are shown in Table 2. Mean FVC, FEV1, TLC, and DLco values were 75.2%, 85.8%, 83.5%, and 62.2%, respectively. Mean resting PaO2 and PaCO2 values were 79.4 and 37.4 mmHg in all patients, respectively.

Full table
Mean FEV1, TLC, and DLco values were significantly different by age group (P<0.001, P=0.010 and P=0.021, respectively) (Table 2). PaO2 and PaCO2 were lower in older patients than younger patients at the initial diagnosis (P<0.001).
Survival analysis
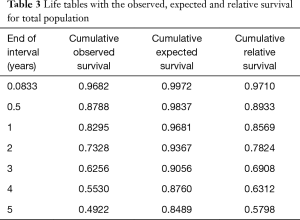
The 1-, 3-, and 5-year observed survival rates were 83.0%, 62.6%, and 49.2% in all 1,663 patients, respectively (Table 3).
Full table
A univariate survival analysis of patients with IPF showed that older age (≥70 years), clinical diagnostic method without surgical lung biopsy, non-treatment, presence of lung cancer, and low initial FVC (% pred) (<60%) were significant poor prognostic factors (Figure 1). The observed survival rate of the ≥70 years of age group was significantly lower than those of other age groups (P<0.001) (Figure 1A).
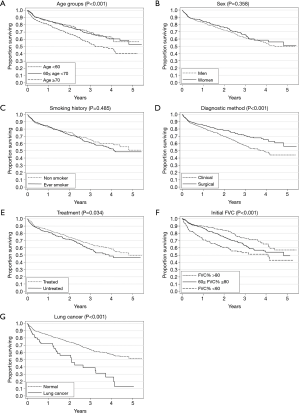
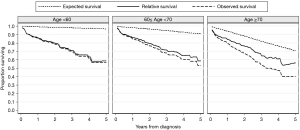
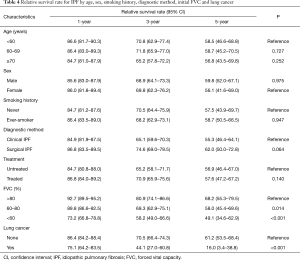
The 1-, 3-, and 5-year relative survival rates were 85.7%, 69.1%, and 58.0% in all patients, respectively (Table 3). Figure 2 shows the expected, observed, and relative survival by age group. The observed survival rate of the group above 70 years of age was significantly lower than those of the other age groups. On the other hand, the relative survival rate results showed quite different trends. No difference in relative survival rate was detected among age groups (Table 4). The 5-year relative survival rate of the group of patients ≥70 years of age was 56.8% and not significantly different from that of the group <60 years of age (56.8%, P=0.252).
Full table
A multivariate analysis revealed low FVC [60–80% (P=0.014), and <60% (P<0.001)], and presence of lung cancer (P<0.001) were significantly associated with low relative survival rate (Table 4).
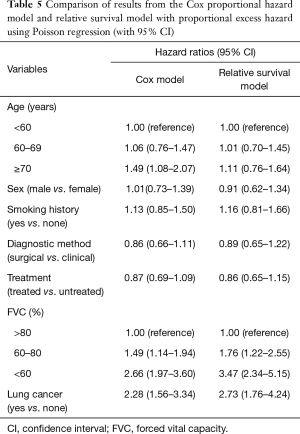
Patients with low FVC (60–80%, and <60%) or lung cancer had lower survival rates according to both the Cox and relative survival models (Table 5). The hazard ratio (HR) for the elderly group (≥70 years) determined by the relative survival model (HR, 1.11) was lower than that determined by Cox model (HR, 1.49). No differences in HR in each age group were observed compared to the reference group (<60 years) in the relative survival analysis.
Full table
Discussion
This study shows that no difference in relative survival rate was detected between the older group (≥70 years) and the younger group (<60 years) (Figure 2), suggesting that the prognosis of older patients is not significantly different than that of younger patients when the effect of age is considered in other causes of death except IPF. Although the observed survival rate of the older group was significantly lower than those of the other groups, it showed a similar survival pattern to that of previous studies (1-6).
King and colleagues (9) suggested a more benign clinical course for younger patients with IPF. Older patients had shorter survival compared with younger patients (<50 years of age, 116.4 months; 50–60 years, 62.8 months; 60–69 years, 27.2 months; ≥70 years, 14.6 months, P<0.0001). However, median survival of young patients was 2.1 years among 22 patients less than 50 years of age in a retrospective study at the Mayo Clinic in 2005 (17). These results indicate that younger patients do not have different prognoses to older patients.
Relative survival rate is the ratio of observed survival rate to expected survival rate. Although the relative survival model is neither new nor statistically superior, it may be an appropriate analysis for the disease-associated mortality associated with age to overcome potential bias associated with age (14). Previous studies (9,17,18) used survival analysis for patients with IPF by analyzing the observed survival time and age-adjusted survival in a multivariate analysis. Relative survival rate has not been used widely in survival analysis of patients with IPF between different age cohorts to detect the effect of age on survival. Two previous studies used relative survival rate to calculate survival of the total population. However, no survival data related to age were provided in these studies (19,20). Thus, this is the first study to use relative survival rate for patients with IPF considering age. Furthermore, this is the first and largest survival analysis study according to age conducted in Asian patients with IPF.
The survival data of patients with IPF are suitable for a relative survival analysis because IPF prevalence is low. If the prevalence of a disease is very low, it will have little impact on the relative survival estimates (14). Relative survival estimates are based on the deaths expected to occur in a cohort of patients with IPF with respect to the general population without the disease. The estimates adjust for the disease-specific mortality associated with age separately from the expected mortality experienced in the general population (14).
The mechanism of effect of aging on survival in patients with IPF is unclear. Age-related changes in cellular function may play roles in IPF (21). Several mutations (mutations in surfactant proteins, gel-forming mucin, and telomerase) suggest a genetic predisposition (22-24), and inflammation and fibroblast and epithelial cell dysfunction are possible pathogenic changes in IPF.
In general, the elder tend to have more combined disease, but, unexpectedly, comorbidities were not different between age groups in the present study (Table 1). And also the percentages of patients with two or more of comorbidities were different between age groups (data are not shown). This finding suggested comorbidities had no effect on the survival.
This study was a broad population-based study. The cause of death is often difficult to establish with certainty due to limited information. The relative survival analysis was not limited because individual cause of death is not required for a relative survival analysis. However, this study had several limitations. First, it was retrospective and re-analyzed Korean National Survey data. Therefore, baseline clinical characteristics differed among age groups, which may have influenced survival. In addition, there may be a difference between the age groups of patients with IPF treated with corticosteroids or cytotoxic therapy that may have affected mortality. To overcome this limitation, we adjusted the survival data by multivariate analysis with risk factors such as diagnostic method, FVC, presence of lung cancer and whether the treatment was done or not. Since the data collected could be analyzed retrospectively, only 74% of the overall cause of death among total 414 deaths was known; hence, we could not analyze the cause of death. We could not describe the proportion of long term oxygen therapy because the use of oxygen therapy was not collected. Second, this study was based on 2002 ATS-ERS guidelines for diagnosing IPF. This population may have different outcomes from those of the current diagnostic guidelines. However, the diagnostic criteria not seemed to affect the purpose of present study to know the effect of age on the mortality. If possible usual interstitial pneumonia (UIP), one of the classified IPF on new diagnostic criteria, were mixed up with definite UIP, it does not affect mortality because of the mortality of definite UIP and possible UIP was not different (25). Third, present study did not show the survival rates in patients less than 50 years of age only. We divided the population into three groups for analysis because the number of patients less than 50 years of age was too small to analyze, and no difference in the survival curve pattern was detected between patients aged less than 50 years and those aged 50–59 years (data are not shown). Fourth, this study included three patients, who were diagnosed by surgical method, aged less than 40 years. The diagnosis of IPF at these ages is extremely rare, and diagnosis could turn to collagen vascular disease associated UIP in the future. However, the pathological findings of IIPF and collagen vascular diseases were different in terms of presence of germinal centers and lymphoid follicles (26). We failed to find out germinal centers and lymphoid follicles in biopsy from patients aged less than 40 years, and then we included these patients the present study. Even if these patients turn to collagen vascular disease associated UIP, the results of this study are less likely affected because of very small size.
In conclusion, the observed survival rate of patients with IPF above 70 years of age was significantly lower than that of patients less than 60 years of age. Relative survival rate of patients with IPF aged above 70 years was not different from that of patients aged less than 60 years. These findings suggest that age may not affect survival in Korean patients with IPF. Further large, prospective studies with a well-organized registry will be needed to assess relative survival in patients with IPF.
Key message: (I) age has no effect on the survival in patients with IPF; (II) the prognosis of patients above 70 years of age with IPF was not different to that of patients less than 60 years of age in terms of relative survival rate.
Acknowledgements
The authors are grateful to all members of the Korean ILD Research Group who helped gather the data for analysis. This work was supported by Soonchunhyang University Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study protocol was reviewed and approved by the Institutional Review Board (IRB) of each hospital, and exempted from approval by the IRB of Soonchunhyang University Seoul Hospital (IRB file number: SCHUH 2014-09-007), which deemed that informed consent was unnecessary.
References
- Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199-203. [Crossref] [PubMed]
- Flaherty KR, Toews GB, Travis WD, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J 2002;19:275-83. [Crossref] [PubMed]
- King TE Jr, Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001;164:1025-32. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J 2002;19:794-6. [Crossref] [PubMed]
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646-64. [Crossref] [PubMed]
- du Bois RM, Weycker D, Albera C, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:459-66. [Crossref] [PubMed]
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- King TE Jr, Tooze JA, Schwarz MI, et al. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171-81. [Crossref] [PubMed]
- Erbes R, Schaberg T, Loddenkemper R. Lung function tests in patients with idiopathic pulmonary fibrosis. Are they helpful for predicting outcome? Chest 1997;111:51-7. [Crossref] [PubMed]
- Sarfati D, Blakely T, Pearce N. Measuring cancer survival in populations: relative survival vs cancer-specific survival. Int J Epidemiol 2010;39:598-610. [Crossref] [PubMed]
- Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Stat Med 2004;23:51-64. [Crossref] [PubMed]
- Micheli A, Coebergh JW, Mugno E, et al. European health systems and cancer care. Ann Oncol 2003;14 Suppl 5:v41-60. [Crossref] [PubMed]
- Nelson CP, Lambert PC, Squire IB, et al. Relative survival: what can cardiovascular disease learn from cancer? Eur Heart J 2008;29:941-7. [Crossref] [PubMed]
- Kim YJ, Park JW, Kyung SY, et al. Clinical characteristics of idiopathic pulmonary fibrosis patients with diabetes mellitus: the national survey in Korea from 2003 to 2007. J Korean Med Sci 2012;27:756-60. [Crossref] [PubMed]
- Lee SH, Kim DS, Kim YW, et al. Association between occupational dust exposure and prognosis of idiopathic pulmonary fibrosis: a Korean national survey. Chest 2015;147:465-74. [Crossref] [PubMed]
- Nadrous HF, Myers JL, Decker PA, et al. Idiopathic pulmonary fibrosis in patients younger than 50 years. Mayo Clin Proc 2005;80:37-40. [Crossref] [PubMed]
- Olson AL, Swigris JJ, Lezotte DC, et al. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 2007;176:277-84. [Crossref] [PubMed]
- Mapel DW, Hunt WC, Utton R, et al. Idiopathic pulmonary fibrosis: survival in population based and hospital based cohorts. Thorax 1998;53:469-76. [Crossref] [PubMed]
- Fernández Pérez ER, Daniels CE, Schroeder DR, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest 2010;137:129-37. [Crossref] [PubMed]
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431-40. [Crossref] [PubMed]
- Crossno PF, Polosukhin VV, Blackwell TS, et al. Identification of early interstitial lung disease in an individual with genetic variations in ABCA3 and SFTPC. Chest 2010;137:969-73. [Crossref] [PubMed]
- El-Chemaly S, Ziegler SG, Calado RT, et al. Natural history of pulmonary fibrosis in two subjects with the same telomerase mutation. Chest 2011;139:1203-9. [Crossref] [PubMed]
- Faner R, Rojas M, Macnee W, et al. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2012;186:306-13. [Crossref] [PubMed]
- Lee JW, Shehu E, Gjonbrataj J, et al. Clinical findings and outcomes in patients with possible usual interstitial pneumonia. Respir Med 2015;109:510-6. [Crossref] [PubMed]
- Song JW, Do KH, Kim MY, et al. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest 2009;136:23-30. [Crossref] [PubMed]


