Current therapy of Eisenmenger syndrome
Eisenmenger syndrome (ES) is a complex and disastrous medical problem with profound cyanosis and clinical deterioration by significant right to left shunting. This syndrome is the most advanced form of pulmonary arterial hypertension (PAH) associated with congenital heart disease (PAH-CHD). Inverted shunt changes structures of pulmonary vasculature like all forms of PAH in ES (1). In the past, the management of patients with ES was limited to conventional therapy with an emphasis on regular informed cardiovascular follow-up. Subsequent clinical studies have made it possible to improve to patient survival and functional capacity. There are major therapeutic targets in PAH treatment using endothelin-receptor antagonists, phosphodiesterase type-5 inhibitors, and prostacyclin derivatives.
In the REVEAL registry, patient’s survival of advanced PAH remains poor despite advanced targeted therapy. Survival rates in newly diagnosed PAH patients after the development of PAH-specific therapies were 86.3% and 61.2% at 1 and 5 years, respectively (2). This result is still disappointing although PAH-CHD shows better outcomes compare to other etiologies of PAH. Nevertheless, many studies suggest advanced PAH therapies should be needed to improve the survival of PAH patients. The BREATHE-5 trial, first placebo-controlled trial in patients with ES, demonstrated a significant improvement of hemodynamics and exercise capacity without adversely affecting systemic arterial oxygen saturation on bosentan-treated patients (3). Other recent randomized controlled trials in ES with phosphodiesterase type-5 inhibitors have shown improvements in exercise capacity and hemodynamics (4,5). In common with other world registries, our group also reports the importance of specialized therapies to improve the survival of PAH patients in Korean Registry of pulmonary arterial hypertension (KORPAH) (Table 1) (6). The demographics of the KORPAH registry participants were similar to those of western registry including 159 patients of PAH-CHD (26.9%) of all patients (n=625) (Figure 1) (6).
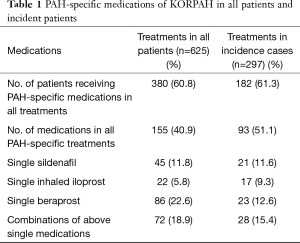
Full table
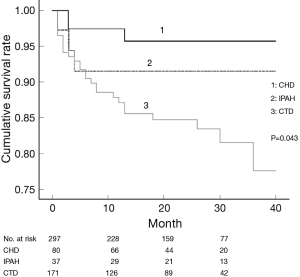
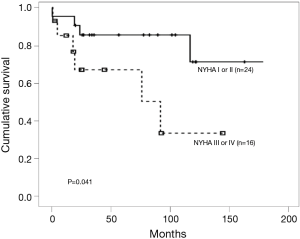
The World Health Organization (WHO) functional classification at the time of diagnosis has major implications as a clinical predictor of mortality and survival for patients with PAH (7). We reported the same results already in the retrospective cohort (Figure 2) (8). The EARLY study, randomized controlled trial of a PAH-targeted therapy in WHO functional class (FC) II patients, suggested that patients with WHO FC II PAH have a severe and often fatally progressive disease (9). These findings demonstrated the importance of the advanced targeted therapy in a timely manner.
Several studies have reported beneficial effects of inhaled iloprost, resulting in improved WHO functional class, hemodynamics, an increased 6-minute walking distance, and quality of life (10,11). A retrospective study of adult patients with PAH to CHD in Korea also suggested that inhaled iloprost as a perioperative medical intervention for patients with PAH-CHD is safe and effective in improving the systemic oxygen saturation and for early recovery in the postoperative course (Table 2) (12). Also, a prospective single-arm study which 18 patients with ES and exertional dyspnea according to WHO functional class III or IV were prospectively recruited had showed that 24 weeks of inhaled iloprost therapy in patients with ES led to significant improvements in exercise capacity, quality of life, and right ventricular function. These results likely explain the symptomatic relief reported by patients with ES receiving iloprost therapy (13).
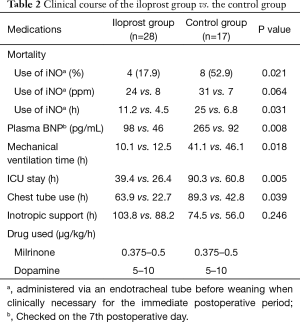
Full table
The recent study of the German National Resister for congenital heart defects (GNR-CHD) contains a nation-wide data with a large population of ES patients in the community (14). Among 153 patients with ES, 57.5% of patients were treated with PAH-specific medical therapies. Of those, 17.6% of patients received combination therapy; 76.1% of patients on monotherapy were on bosentan and 44.4% of patients treated primarily with Sildenafil were also on this drug as a second line. The GNR-CHD is a well-designed study and recruited a large number of patients representing the community-based population. The result of this study may be valuable data on advanced targeted therapy. However, as the intrinsic drawback of registry, the lack of uniform treatment strategy couldn’t explain effective and timely treatments in PAH-targeted therapy. This limitation was shown as no outcome difference between monotherapy and dual targeted therapy because of the long escalation time. Nevertheless, the strongest message from GNR-CHD is the better clinical outcome in the large volume centers than remaining centers that means the need of expert care from centers of excellence.
In summary, the GNR-CHD demonstrated better survival of advanced targeted therapy based on the real world as well as tertiary referrals in the Germany. This modern real world registry data reinforce the reason why specialized medical therapies in PAH expert center should be considered in ES patients.
Acknowledgements
Funding: This research was partly supported by the Gachon University Gil Medical Center (Grant number: 2015-02) and the Next-generation Medical Device Development Program for Newly-Created Market of the National Research Foundation (NRF) funded by the Korean government, MSIP (No. 2015M3D5A1066043).
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Haiyun Yuan (Department of Cardiovascular Surgery, Guangdong Provincial Cardiovascular Institute, Guangdong General Hospital, Guangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pietra GG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 2004;43:25S-32S. [Crossref] [PubMed]
- Farber HW, Miller DP, Poms AD, et al. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest 2015;148:1043-54. [Crossref] [PubMed]
- Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006;114:48-54. [Crossref] [PubMed]
- Mukhopadhyay S, Nathani S, Yusuf J, et al. Clinical efficacy of phosphodiesterase-5 inhibitor tadalafil in Eisenmenger syndrome--a randomized, placebo-controlled, double-blind crossover study. Congenit Heart Dis 2011;6:424-31. [Crossref] [PubMed]
- D'Alto M, Romeo E, Argiento P, et al. Bosentan-sildenafil association in patients with congenital heart disease-related pulmonary arterial hypertension and Eisenmenger physiology. Int J Cardiol 2012;155:378-82. [Crossref] [PubMed]
- Chung WJ, Park YB, Jeon CH, et al. Baseline characteristics of the Korean Registry of Pulmonary Arterial Hypertension. J Korean Med Sci 2015;30:1429-38. [Crossref] [PubMed]
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev 2010;19:272-8. [Crossref] [PubMed]
- Park YM, Chung WJ, Choi DY, et al. Functional class and targeted therapy are related to the survival in patients with pulmonary arterial hypertension. Yonsei Med J 2014;55:1526-32. [Crossref] [PubMed]
- Simonneau G, Galiè N, Jansa P, et al. Long-term results from the EARLY study of bosentan in WHO functional class II pulmonary arterial hypertension patients. Int J Cardiol 2014;172:332-9. [Crossref] [PubMed]
- Ivy DD, Doran AK, Smith KJ, et al. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol 2008;51:161-9. [Crossref] [PubMed]
- Opitz CF, Wensel R, Winkler J, et al. Clinical efficacy and survival with first-line inhaled iloprost therapy in patients with idiopathic pulmonary arterial hypertension. Eur Heart J 2005;26:1895-902. [Crossref] [PubMed]
- Sung KW, Jeon YB, Kim NY, et al. The effects of perioperative inhaled iloprost on pulmonary hypertension with congenital heart disease. Cardiology 2013;126:224-9. [Crossref] [PubMed]
- Cha KS, Cho KI, Seo JS, et al. Effects of inhaled iloprost on exercise capacity, quality of life, and cardiac function in patients with pulmonary arterial hypertension secondary to congenital heart disease (the Eisenmenger syndrome) (from the EIGER Study). Am J Cardiol 2013;112:1834-9. [Crossref] [PubMed]
- Diller GP, Körten MA, Bauer UM, et al. Current therapy and outcome of Eisenmenger syndrome: data of the German National Register for congenital heart defects. Eur Heart J 2016;37:1449-55. [Crossref] [PubMed]


