The diagnosis efficacy and safety of video-assisted thoracoscopy surgery (VATS) in undefined interstitial lung diseases: a retrospective study
Introduction
Interstitial lung disease (ILD) refers to a group of lung diseases with a great diversity regarding to etiology, pathological change, treatment and prognosis. It is therefore important to validate the specific diagnosis and classification of ILD for the treatment options and prognosis evaluation. The current classification of ILD and guidelines emphasize the significant role of surgical lung biopsy (SLB) for the definite diagnosis of ILD but also encourage physicians to balance the benefit against risks of performing the surgery (1,2).
In recent years, the necessity of SLB has been questioned due to the development of high-resolution computerized tomography (HRCT) and transbronchial lung biopsies (TBLB) via bronchoscopy (3). Although the diagnosis could be established with clinical information, detailed lab testing, high-quality HRCT (4) and TBLB with the consultation of pulmonologist, radiologist and pathologist working together in a majority of cases (5), it was shown that the sensitivity and specificity of this approach for the diagnosis of idiopathic pulmonary fibrosis (IPF) are within the 60-80% range (6). Moreover, TBLB may not be sufficient for the definite pathological diagnosis due to the amount and site of biopsies. A retrospective study in 21 IPF patients found that only 32% patients could be accurately diagnosed with tissue obtained by TBLB, compared to 95.4% by SLB (7).
There have been reports on the contribution of SLB to the final diagnosis and appropriate treatment of ILD. A retrospective study in 80 unclassified ILD patients found that approximately 40% of them were eventually diagnosed as IPF with SLB (8). Another retrospective study in 61 unclassified ILD patients reported that definite diagnosis was obtained in 94.1% patients and treatment was changed for 87% patients after an SLB (9). Parambil et al. demonstrated that SLB could identify causes for 87% diffuse alveolar damage (DAD) that manifested as diffuse lung infiltrates radiologically, thereby improving the treatment efficacy and reducing the mortality of patients (10).
According to recent studies the incidence of post-SLB complications was 16-71% (11-13), and the overall post-operative mortality was 4.5-6% (8,11). It was reported that decreased pulmonary diffusion function (diffusing capacity of the lungs for carbon monoxide, DLCO <50% predicted value), requiring mechanical ventilation (MV), immuno-compromised status and pulmonary hypertension may be associated with increased risk of death after SLB. In selected patients who had no risk factors, the overall mortality rate in 90-day post-SLB was reported to be 1.5% (8). Moreover, the shift from conventional thoracotomy toward video-assisted thoracoscopy surgery (VATS) favors the new technique in regards to complications and mortality (14,15).
The main objectives of this study were to explore the diagnostic yield and safety of VATS in unclassified ILD, and the risk factors associated with post-operative complications.
Patients and methods
Patients selection
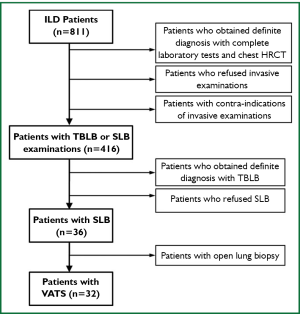
Among the 811 ILD patients admitted into the First Affiliated Hospital of Guangzhou Medical College from January 1, 2007 to December 31, 2011, those received VATS lung biopsy due to unclassified ILD even after the diagnosis procedure recommended by the guideline (Figure 1). The diagnosis procedure included team work of pulmonologist, radiologist and pathologist with detailed clinical information, complete laboratory test, pulmonary function tests, chest HRCT and TBLB examinations. Subjects who required continuous mechanical ventilation and whose DLCO <50% predicted were excluded.
Data collection
Clinical information was collected including age, sex, body mass index (BMI), smoking index, disease history, medication, physical examination and vital signs, arterial gas test results, detailed lab tests, pulmonary function results, diagnosis before and after VATS. The VATS was performed under the guidance of the chest HRCT with detailed record of biopsy number and site, anesthesia mode, duration of the chest drainage, post-operative complications, and 30- and 90-day mortality rates. The final diagnosis was determined by 2 respiratory physicians, 1 radiologist and 1 pathologist.
The statistical analysis was carried out with SPSS16.0 software package. The continuous variables were expressed as means and the categorical variables as percentages. The comparisons were performed with the unpaired t test and chi-squared test where appropriate. P<0.05 was regarded as statistically significant.
Results
A total of 811 admitted patients were diagnosed as ILD during a 5-year period, among whom 416 (51.3%) accepted invasive examinations, including TBLB and SLB. SLB was performed in 36 (4%) patients, including 32 (3.9%) accepted VATS. These 32 cases with VATS were included in the study.
There were 20 (62.5%) males and 12 (37.5%) females. The mean age was 52.2 years (30-76 yrs) and mean BMI was 23.7 (18.0-30.3). Eleven (34.4%) patients had a history of smoking and 8 (25.0%) were heavy smokers (smoking index more than 20 pack years). Five (15.6%) patients took immunosuppressive drug and 21 (65.6%) steroid. The results of arterial gas test before the VATS were the following: PaO2: 78.4 mmHg (49.1-121.1 mmHg), PaCO2: 37.52 mmHg (30.5-45.1 mmHg).
The predominant abnormal areas on chest HRCT were lower lobes (50%), followed by randomized distribution (34.4%) and upper lobes (15.6%). The characteristic HRCT features were ground glass attenuation (87.5%), reticular lines (65.6%), patchy consolidation (43.8%), bullae (25%), pleural thickening (21.9%), honeycombing (21.9%), subpleural lines (18.8%), patchy nodules (12.5%), emphysema (6%), pleural effusion (6%) and mediastinal lymph nodes enlargement (6%).
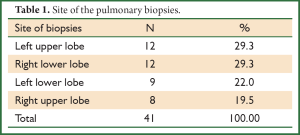
Among 32 patients, general anesthesia was performed in 29 (90.6%) and local anesthesia in 3 (9.4%) patients. A total of 41 biopsy samples were obtained by VATS, nine (28.1%) patients with double biopsies from the same lobes and 23 (71.9%) with only a single biopsy. The detailed sites of the VATS were summarized in Table 1.
Full Table
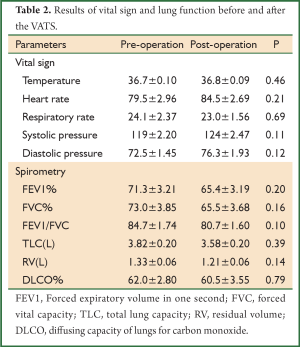
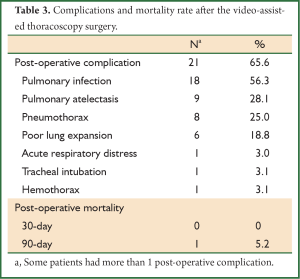
The mean duration of the chest drainage was 2.72 days [0-8 days] after the VATS, and in 5 (15.6%) patients, the chest drain could be withdrawn right after the surgery. The parameters of the vital sign were not significantly changed while there was a non-significant decrease in pulmonary function parameters 2-3 weeks after the VATS (Table 2). Post-operative complications were reported in 21 (65.6%) patients, including pulmonary infection, pulmonary atelectasis, pneumothorax, acute respiratory failure, tracheal intubation and hemothorax. No patient died within 30 days after VATS, while 1 patient died within 90 days due to pulmonary infection with no complications reported immediately after the VATS (Table 3).
Full Table
Full Table
Patients were divided into 2 groups based on the existence of post-operative complications, and there was no significant risk factor contributing to the prevalence of complication, including age, BMI, smoking index, lung function, anesthesia method, the time of thoracic drain withdrawn and the use of immunosuppressive drugs or steroids.
All the 32 patients obtained definite diagnosis after the VATS. The diagnosis was changed in 27 (84.4%) and unchanged in 5 (15.6%) patients. Table 4 compared the pre- and post-operative diagnosis. Among 20 cases (62.5%) diagnosed as unclassified ILD before the surgery, 14 (70.0%) were diagnosed as nonspecific interstitial pneumonia (NSIP), 3 (15.0%) as idiopathic pulmonary fibrosis (IPF) and 3 (15.0%) as connective tissue disease-related ILD (CTD-ILD). Among the 7 cases with complete change after VATS, 4 (57.1%) were cryptogenic organizing pneumonia (COP).

Full Table
Discussion
ILD comprises a heterogeneous group of lung diseases with diverse etiologies, pathological changes, response to treatments and prognoses. The development of chest HRCT and TBLB techniques greatly improve the ability of diagnosis of ILD (16,17). However, clinical and radiological data may not be sufficient for some patients without definitive environmental exposure and manifestations of systemic diseases (6). Although TBLB is useful in some cases, it may be of less efficacy in establishing diagnosis due to the limit of the amount biopsy sampling (7).
Currently, the role of SLB in the diagnosis of ILD is still controversial. Despite the advent of VATS for lung biopsy and the progress of post-surgery intensive care, many physicians are overcautious on the balance between the efficacy of VATS on diagnosis and the risk of SLB (18).
In this study, among a total of 811 patients diagnosed as ILD during a 5-year period, only 32 (3.9%) patients accepted VATS. In these selected ILD patients, the diagnosis was changed from the previous diagnosis in 84.4% after SLB. The results are relatively higher than those published in the literature (8-10,17). It should be emphasized that all patients included in the study had uncertain diagnosis even after strict diagnostic procedures (including TBLB) with the consultation of pulmonologist, radiologist and pathologist working together. We believe that these might be the candidates demanding SLB for definite diagnosis, although there is no consensus on the indication for SLB for ILD patients yet.
Based on the chest HRCT features, we found that 28 (87.5%) patients manifested as ground glass attenuation, 21 (65.6%) as reticular lines, and 14 (43.8%) as patchy consolidation, indicating that atypical radiological manifestations may be one of the important reasons for the surgery procedure. Moreover, in 7 patients that the final diagnosis was completely changed after the VATS, 4 were diagnosed as COP manifesting as patchy consolidation or nodules in chest HRCT, indicating that suspected COP patients with nonspecific presentations may be good candidates for VATS.
It was reported that the site and number of biopsies may affect the diagnostic efficacy of VATS. Gaensler et al. suggested that the lingula and middle lobes are not representative and should be avoided for the biopsy (19), while Morell et al. found that the diagnoses from the lingula and middle lobes coincided with those from other lobes (20). In the present study with 41 biopsies, the site of biopsy was determined by the abnormalities on CT scan with one biopsy site in majority of the cases and no biopsy obtained from the lingula or middle lobe, which were similar to the data published by Fibla (17). It was found that a single site of biopsy may be sufficient to obtain the definite diagnosis for most patients (71.9%).
The most common final diagnosis in 32 patients was NSIP (50%), followed by IPF (12.5%). The results was similar to those published in China (9), although there were reports on patients abroad with different results showing that IPF was the most common cause of unclassified ILD requiring SLB (26-30%) (17). The explanation for this difference may be as following. (I) The categorical distribution of ILD is different in China from abroad where the epidemiological data of ILD cited IPF as the most common cause (21); (II) There may be some undifferentiated connective tissue diseases diagnosed as NSIP, while the sophisticated rheumatology abroad may be more likely able to distinguish these patients at an early stage and preclude the SLB (18,22); (III) The small sample size of this study may not be powerful enough to represent the conditions of other hospitals and other area. Therefore, the cooperation with different disciplines including the rheumatology and multi-center studies nationwide may further demonstrate the indications and diagnostic efficacy of SLB in unclassified ILD in the future.
The incidence of post-VATS complications was reported to be 16-71%, including pulmonary infection, pneumothorax, tracheal intubation, acute respiratory distress and prolonged air leakage (11-13). In this study, 65.6% patients presented the post-operative complications, and the most common one were pulmonary infection (56.3%), followed by pulmonary atelectasis (28.1%) and pneumothorax (25.0%). These results indicated that although there were relatively high post-VATS complication, majority of them were infection related and could be controlled by effective antibiotic treatment. The risk factors of the post-operative complications included for analysis were age, BMI, smoking index, lung function, anesthesia method, the time of thoracic drain withdrawn and the use of immunosuppressive drugs or steroids. None was found to be associated with the occurrence of these complications, probably due to limited cases included in the present study.
The data is sparse regarding the effect of VATS on the lung function of patients. We found that there was a trend of decrease in the pulmonary function (FEV1%, FVC%, FEV1/FVC, TLC, and DLCO%) after the VATS, suggesting that the surgery may have mild adverse effects on lung function although no statistical significance was found in the present study. The parameters of vital sign also recovered to the basal level 2-3 weeks after the VATS. This may be due to the fact that the VATS technique is minimal invasive as compared with conventional, and the recovery from VATS is quite well with the modern intensive care after the surgery. Once again, the power of the study is not enough due to relatively small sample size.
Post-operative mortality is another essential component regarding to the benefit-risk balance. In the literature, a meta-analysis of 2,223 cases demonstrated that the 30-day mortality after the SLB was 4.5% (3.7-5.5%) (11), and Lettieri et al. reported that the 90-day mortality rate was 6% (8). Continuous mechanical ventilation, DLCO less than 50% predicted value, immunosuppressive status and pulmonary hypertension were suggested to be the risk factors of increased mortality. Excluding patients who met either criterion rendered a decrease of 90-day post-SLB mortality rate to 1.5% (8). In our study, the VATS is a safe procedure with a 30- and 90-day post-VATS mortality of 0 and 5.2%, respectively. The low mortality rate may be related to patients selection, expertise in VATS and post-operation intensive care. All the subject in the present study were without risk factors mentioned above.
In summary, this retrospective analysis showed that VATS was a powerful diagnostic tool for ILD patients who were unclassified even after the consultation of pulmonologist, radiologist and pathologist working together and it is relatively safe with appropriate patients selection, expertise in VATS and post-operation intensive care. Prospective multi-center study of larger scale is necessary in the future to further determine the appropriate patients selection criteria for VATS for diagnosis of ILD with good balance between the efficacy and risk.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J 2002;19:794-6. [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [PubMed]
- Halkos ME, Gal AA, Kerendi F, et al. Role of thoracic surgeons in the diagnosis of idiopathic interstitial lung disease. Ann Thorac Surg 2005;79:2172-9. [PubMed]
- Flaherty KR, King TE Jr, Raghu G, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med 2004;170:904-10. [PubMed]
- Flaherty KR, Andrei AC, King TE Jr, et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med 2007;175:1054-60. [PubMed]
- Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164:193-6. [PubMed]
- Berbescu EA, Katzenstein AL, Snow JL, et al. Transbronchial biopsy in usual interstitial pneumonia. Chest 2006;129:1126-31. [PubMed]
- Lettieri CJ, Veerappan GR, Helman DL, et al. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest 2005;127:1600-5. [PubMed]
- Ye Q, Dai H, Huang H. Respiratory critical care medicine: 2010-2011. People’s Medical Publishing House, 2011.
- Parambil JG, Myers JL, Aubry MC, et al. Causes and prognosis of diffuse alveolar damage diagnosed on surgical lung biopsy. Chest 2007;132:50-7. [PubMed]
- Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg 2007;83:1140-4. [PubMed]
- Sigurdsson MI, Isaksson HJ, Gudmundsson G, et al. Diagnostic surgical lung biopsies for suspected interstitial lung diseases: a retrospective study. Ann Thorac Surg 2009;88:227-32. [PubMed]
- Yang W, He B. Complications of lung biopsy in patients with idiopathic interstitial pneumonia and risk factors thereof. Zhonghua Yi Xue Za Zhi 2009;89:109-13. [PubMed]
- Ravini M, Ferraro G, Barbieri B, et al. Changing strategies of lung biopsies in diffuse lung diseases: the impact of video-assisted thoracoscopy. Eur Respir J 1998;11:99-103. [PubMed]
- Tiitto L, Heiskanen U, Bloigu R, et al. Thoracoscopic lung biopsy is a safe procedure in diagnosing usual interstitial pneumonia. Chest 2005;128:2375-80. [PubMed]
- Dixon S, Benamore R. The idiopathic interstitial pneumonias: understanding key radiological features. Clin Radiol 2010;65:823-31. [PubMed]
- Fibla JJ, Molins L, Blanco A, et al. Video-assisted thoracoscopic lung biopsy in the diagnosis of interstitial lung disease: a prospective, multi-center study in 224 patients. Arch Bronconeumol 2012;48:81-5. [PubMed]
- Sun YC. Surgical lung biopsy in the diagnosis of idiopathic interstitial pneumonias. Zhonghua Jie He He Hu Xi Za Zhi 2007;30:243-5. [PubMed]
- Gaensler EA, Carrington CB. Open biopsy for chronic diffuse infiltrative lung disease: clinical, roentgenographic, and physiological correlations in 502 patients. Ann Thorac Surg 1980;30:411-26. [PubMed]
- Morell F, Reyes L, Doménech G, et al. Diagnoses and diagnostic procedures in 500 consecutive patients with clinical suspicion of interstitial lung disease. Arch Bronconeumol 2008;44:185-91. [PubMed]
- Ishie RT, Cardoso JJ, Silveira RJ, et al. Video-assisted thoracoscopy for the diagnosis of diffuse parenchymal lung disease. J Bras Pneumol 2009;35:234-41. [PubMed]
- Huang H, Xu ZJ, Zhu YJ, et al. Clinical analysis of different pathological patterns of nonspecific interstitial pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2006;29:747-50. [PubMed]


