Pemetrexed-induced radiation recall dermatitis in a patient with lung adenocarcinoma: case report and literature review
Introduction
Radiation recall dermatitis (RRD) is an inflammatory reaction in a previously irradiated field subsequent to the administration of pharmacologic or promoting agents. Although RRD affects the skin, the radiation recall phenomenon can affect internal soft tissues and organs, including the lungs. RRD precipitated by pemetrexed, a third-generation multitargeted antifolate compound approved and used in the treatment of malignant pleural mesothelioma and NSCLC (1-3), is a rarely reported phenomenon (4-8). We describe a 47-year-old man with NSCLC who was treated with radiotherapy and pemetrexed-based chemotherapy 3 years prior to developing RRD upon resumption of pemetrexed-based chemotherapy.
Case presentation
A 47-year-old male never-smoker initially presented in February 2010 with a 15-month history of intermittent hemoptysis. Thoracic CT demonstrated a 7.0 cm × 5.7 cm × 5.5 cm left upper lobe mass extending into the anterior pleural surface and mediastinum with multiple bilateral pulmonary nodules. Biopsy demonstrated an EGFR-mutated moderately differentiated adenocarcinoma. Brain MRI revealed three metastases (left parietal lobe, two in the right cerebellum).
Initially, Gamma Knife stereotactic radiosurgery was delivered to each brain lesion (21 Gy), followed by external beam radiotherapy to the lung mass (60 Gy in 30 fractions, 3D conformal technique) with concurrent carboplatin and pemetrexed from March 2010 to April 2010. Definitive therapy was performed given the EGFR-positive nature of the disease together with young age and good performance status. By the final 2 weeks of radiotherapy, the patient had developed a Grade 2 [by the Common Toxicity Criteria for Adverse Events (CTCAE) scale] skin reaction localized to his left upper back, most notable over the medial border of the scapula. He continued adjuvant carboplatin and pemetrexed for a total of six cycles (completed July 2010). This was followed by maintenance pemetrexed alone for six further cycles from July to November 2010, with interval resolution of the skin reaction. Imaging during the following three years showed intermittent tumor regression followed by progressive disease, for which the patient received numerous systemic regimens (in order of administration): erlotinib, cetuximab and afatinib, bevacizumab with daily erlotinib, bevacizumab with weekly erlotinib, and a combination of erlotinib, bevacizumab, carboplatin, and paclitaxel. Throughout treatment, the patient remained active and maintained a high functional status.
Subsequently, imaging in February 2013 showed further disease progression. Thirty-four months after completion of initial radiotherapy, the patient was restarted on pemetrexed 500 mg/m2 every 3 weeks with dexamethasone and folic acid. Seven days after the first infusion, the patient developed new cutaneous erythema confined solely to his left upper back, identical in location and similar in intensity to the erythema he experienced at the end of his initial course of thoracic radiotherapy. The affected area consisted of a confluent erythematous rash with superficial edema without papules, vesicles, or desquamation (Figure 1A).
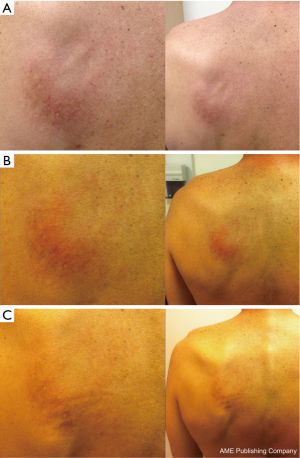
At the time of presentation of this skin reaction, there were no new medications; concomitant medications included aprepitant, vitamin B complex-biotin-folic acid, dronabinol, esomeprazole, gabapentin, hyoscyamine sulfate, methylphenidate, omega-3 fatty acid, and vitamin D-cholecalciferol. A clinical diagnosis of RRD with Grade 2 toxicity (per CTCAE version 4) was then made. The RRD was managed symptomatically with topical moisturizers and steroids. Re-challenge with subsequent pemetrexed infusions did not exacerbate the erythema (Figure 1B,C). Due to the stability of the RRD and the absence of any systemic radiation recall reaction, pemetrexed was continued with close monitoring.
Discussion
The current case report is only the sixth known case of RRD induced by pemetrexed, with only three prior cases of RRD induced by pemetrexed in patients with NSCLC (Table 1). Additionally, the incident case is the first reported case of pemetrexed-induced RRD in North America.
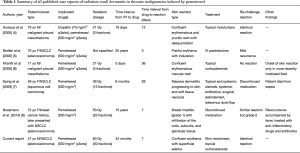
Full table
Pemetrexed, an antifolate compound approved for the treatment of malignant pleural mesothelioma non-squamous NSCLC (1-3), can commonly produce myelosuppression, constitutional symptoms, gastrointestinal dysfunction, dyspnea, chest pain, and cutaneous reactions (9). In clinical practice, the dose-limiting toxicities of pemetrexed include myelosuppression and decreased renal function, especially when used in combination with cisplatin. The rate of all cutaneous adverse reactions in radiation-naïve patients range from 1.4% to as high as 38%, and steroid prophylaxis is sometimes administered before pemetrexed infusions (1-3). Severe cutaneous adverse reactions, defined as CTCAE Grades 3 or 4, occur in up to 6% of patients. Cutaneous adverse reactions, however, are commonly referred to as “rash” in clinical trials, without any specifics of the actual pathology or etiology. In case reports, pemetrexed-related cutaneous adverse events have been described as acute generalized exanthematous pustulosis (10), toxic epidermal necrolysis (11), and RRD (4-8).
Although well-described and likely underreported, the incidence of RRD is thought to be rare. RRD occurs when a precipitating agent, such as a cytotoxic drug, is administered after radiation therapy. RRD is a nondescript term that encompasses a diverse set of pathologies ranging from maculopapular eruptions with erythema, vesicle formation, desquamation, to severe skin necrosis. Though radiation recall mucositis, pneumonitis, colitis, myositis, vulvovaginitis, and optic neuritis have been described (12-16), RRD is the most common form of inflammatory reactions of tissues in prior radiation fields. Mechanisms could be related to increased sensitivity to cytotoxic drugs brought on by radiotherapy-induced vascular damage or stem cell depletion; alternatively, radiation exposure may decrease the immunocompetence of affected tissue, leading to “idiosyncratic drug hypersensitivity” in certain tissues (17).
In addition to pemetrexed, other drugs, most notably gemcitabine, have been associated with RRD in the treatment of other thoracic malignancies. Radiotherapy dosages associated with RRD in these thoracic cases have ranged from 20 to 65 Gy, with a median dose of 39 Gy. Yeo et al. suggested the existence of a minimal threshold radiation dosage needed for a recall reaction to occur (18). In the present case, the RRD clinical focus was at the skin overlying the medial left scapular border, where treatment planning software predicted a local hotspot dose of 58.5 Gy. Interestingly, the patient did not have a recall reaction on the anterior chest wall, where the maximum dosage was 34.0 Gy. Spirig et al. also showed a minimal threshold dose that manifested in the RRD localizing to the patient’s back and sparing the front side of the radiation portal (7).
The time interval between radiotherapy and drug exposure in RRD in thoracic malignancies can vary from days to decades, consistent with non-thoracic cases of RRD. We found the median time interval between radiotherapy and drug exposure for RRD in thoracic malignancies to be 20 days, consistent with findings by Hird et al. that most reactions occur between a <2 months interval between radiotherapy and drug administration (19). Also posited is that more severe skin reactions occur with shorter intervals. However, intervals less than 7 days may represent radiation sensitization rather than a true recall reaction (17). In two of the six case reports of pemetrexed-related RRD, the time interval between radiotherapy and chemotherapy was 5 days (4,6). It is possible that these cases may have been radiation sensitization rather than true recall reactions. In our present case, the time interval between radiotherapy and pemetrexed was 34 months.
Likewise, rapidity of symptom onset from drug exposure can also vary greatly in RRD. We found the time to RRD onset in the treatment of thoracic malignancies ranged from 18 hours to 42 days, with a median time of 12 days. The variable speed of symptom onset indicates that a pre-sensitization phenomenon may be involved with the development of the recall reaction (17). In the present case, the patient’s initial concurrent chemoradiation course in 2010 was likely the pre-sensitization impetus. Re-challenge with pemetrexed produced an identical reaction as what occurred in this patient three years earlier, but the response to drug re-exposure is variable in the literature. Re-challenge reactions in thoracic malignancies have ranged from no reaction to severe exacerbation. In addition, it has been postulated that RRD is extremely drug-specific. Patients with a history RRD to one cytotoxic agent are not thought to be at increased risk of developing a similar recall reaction upon exposure to a second potentially RRD-inducing agent (17). A definitive link to other hypersensitivity and/or autoimmune disorders has also not been established. In our case, the patient also received erlotinib at several points during his treatment history, but did not produce a RRD in response to the tyrosine kinase inhibitor. This is consistent with the report by Barlési et al., in which a patient did not have recall or cutaneous reactions to the initial chemotherapy regimen of gemcitabine and carboplatin but did upon initiation of second-line pemetrexed (5).
Importantly, RRD may serve as the most visible sign of recall affecting internal organs. In five previous cases of RRD in thoracic malignancies, RRD was accompanied by clinical and/or radiographic evidence of internal organ involvement. Thus, when providers are confronted with possible RRD in thoracic malignancies, it may be prudent to screen for respiratory symptoms to avoid acute decompensations. In the current case of pemetrexed-induced RRD, our patient exhibited no clinical symptoms of respiratory compromise. In addition, the dermatitis was relatively mild and did not worsen upon further cycles of pemetrexed. The patient was, therefore, managed conservatively. In previous case studies, systemic corticosteroids, antibiotics, antihistamines, and NSAIDs have also all been utilized as treatments for RRD. In severe cases of skin and soft tissue necrosis, surgical debridement and reconstruction are necessary. Depending on the severity of RRD, the offending agent may need to be discontinued.
Conclusions
Although RRD is a rare phenomenon, it should be considered in any patient with dermatologic reactions that occur at the site of previous exposure to radiation therapy. When severe, RRD can pose a significant, and possibly life-threatening, impediment to the treatment and care of oncology patients. Moreover, RRD may be a harbinger of internal organ involvement, such as pneumonitis in thoracic oncology patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hazarika M, White RM Jr, Booth BP, et al. Pemetrexed in malignant pleural mesothelioma. Clin Cancer Res 2005;11:982-92. [PubMed]
- Cohen MH, Johnson JR, Wang YC, et al. FDA drug approval summary: pemetrexed for injection (Alimta) for the treatment of non-small cell lung cancer. Oncologist 2005;10:363-8. [Crossref] [PubMed]
- Cohen MH, Justice R, Pazdur R. Approval summary: pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer. Oncologist 2009;14:930-5. [Crossref] [PubMed]
- Hureaux J, Le Guen Y, Tuchais C, et al. Radiation recall dermatitis with pemetrexed. Lung Cancer 2005;50:255-8. [Crossref] [PubMed]
- Barlési F, Tummino C, Tasei AM, et al. Unsuccessful rechallenge with pemetrexed after a previous radiation recall dermatitis. Lung Cancer 2006;54:423-5. [Crossref] [PubMed]
- Khanfir K, Anchisi S. Pemetrexed-associated radiation recall dermatitis. Acta Oncol 2008;47:1607-8. [Crossref] [PubMed]
- Spirig C, Omlin A, D'Addario G, et al. Radiation recall dermatitis with soft tissue necrosis following pemetrexed therapy: a case report. J Med Case Rep 2009;3:93. [Crossref] [PubMed]
- Boesmans S, Decoster L, Schallier D. Pemetrexed-induced radiation recall dermatitis of the breast. Anticancer Res 2014;34:1179-82. [PubMed]
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett 2011;2:769-772. [PubMed]
- Bracke A, Van Marck E, Lambert J. Acute generalized exanthematous pustulosis after pemetrexed, and recurrence after re-introduction. Clin Exp Dermatol 2009;34:337-9. [Crossref] [PubMed]
- Bosch-Barrera J, Gaztañaga M, Ceballos J, et al. Toxic epidermal necrolysis related to pemetrexed and carboplatin with vitamin B12 and folic acid supplementation for advanced non-small cell lung cancer. Onkologie 2009;32:580-4. [Crossref] [PubMed]
- Donaldson SS, Glick JM, Wilbur JR. Letter: Adriamycin activating a recall phenomenon after radiation therapy. Ann Intern Med 1974;81:407-8. [Crossref] [PubMed]
- Schweitzer VG, Juillard GJ, Bajada CL, et al. Radiation recall dermatitis and pneumonitis in a patient treated with paclitaxel. Cancer 1995;76:1069-72. [Crossref] [PubMed]
- Jeter MD, Jänne PA, Brooks S, et al. Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys 2002;53:394-400. [Crossref] [PubMed]
- Hattangadi J, Esty B, Winey B, et al. Radiation recall myositis in pediatric Ewing sarcoma. Pediatr Blood Cancer 2012;59:570-2. [Crossref] [PubMed]
- Gabel C, Eifel PJ, Tornos C, et al. Radiation recall reaction to idarubicin resulting in vaginal necrosis. Gynecol Oncol 1995;57:266-9. [Crossref] [PubMed]
- Camidge R, Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol 2001;59:237-45. [Crossref] [PubMed]
- Yeo W, Leung SF, Johnson PJ. Radiation-recall dermatitis with docetaxel: establishment of a requisite radiation threshold. Eur J Cancer 1997;33:698-9. [Crossref] [PubMed]
- Hird AE, Wilson J, Symons S, et al. Radiation recall dermatitis: case report and review of the literature. Curr Oncol 2008;15:53-62. [Crossref] [PubMed]


