Comparison of the prognostic values of various nutritional parameters in patients with esophageal squamous cell carcinoma from Southern China
Introduction
Cancer has become a critical health problem and a leading cause of death worldwide (1-4). Cancer-related undernutrition, such as malnutrition and weight loss, has been reported frequently in patients with malignant neoplastic disease (5,6). Impaired nutrition status would increase the risk of antitumor treatment, prolong the hospital stay, deteriorate the quality of life and therefore influence the clinical outcome. Assessment of baseline nutritional status has become a novel method to evaluate the prognosis, using a series of nutritional indicators such as body mass index (BMI) (7,8), prognostic nutritional index (PNI) (9-12) and et al., in patients with head and neck cancer, gastric cancer, breast cancer, prostate cancer and pancreatic cancer.
Esophageal cancer is a common malignancy with a high burden of morbidity and mortality (4). Compared with other digestive cancers, the nutritional condition of patients with esophageal cancer is relatively poorer, with more than 70% of them suffering from undernutrition at initial diagnosis (13). Among these patients with esophageal cancer, reduced food intake mostly because of dysphagia always leads to malnutrition, and additional energy demands of systematic inflammation further aggravates weight loss and anorexia. The underlying correlation between nutritional status and risk of postoperative complications has long been discussed, while the role of nutritional intervention for malnourished patients with esophageal cancer is identified (14). However, baseline nutritional condition has received minor attention in risk stratification for patients with esophageal cancer.
During the last decade, several studies have explored the prognostic impact of baseline nutritional status mostly identified by BMI on the outcome of esophageal cancer patients (15-19). In these studies, most of which focused on the adenocarcinoma patients undergoing surgical resection, didn’t come to an agreement. Furthermore, results from Asia, where the esophageal squamous cell carcinoma (ESCC) predominates, were rarely reported thus far.
Therefore, we conducted this clinical study to investigate the prognostic effect of baseline nutrition status in a consecutive cohort of ESCC patients from Southern China. In order to screen out the most appropriate indicator for nutritional assessment, we enrolled the BMI, PNI, Broca Index, ideal bodyweight (IBW) and body weight change to identify and compare their values in predicting the clinical outcomes of ESCC patients. We sought to provide a novel prognostic method for ESCC patients.
Materials and methods
Ethics statement
All patients provided authorized written informed consent for their information to be stored in Sun Yat-Sen University Cancer Center database and to be used for the research. Study approval was obtained from independent ethics committees at Cancer Center of Sun Yat-Sen University. The study was undertaken in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Patients
Between January 2007 and December 2008, five hundred and two patients who attended Sun Yat-Sen University Cancer Center were retrospectively analyzed. All the patients enrolled in this study were pathologically diagnosed as ESCC and received treatment at our center. Detailed medical records with basic demographics (gender, age), detailed medical history and medications, as well as patients’ baseline tumor characteristics (grade of tumor differentiation and stage by the 6th edition of AJCC/UICC TNM system) were collected for subsequent analysis. When the treatment finished, each patient was followed up every three months by telephone contact for at least 5 years. The last follow-up time was January 31st 2012.
Nutritional parameters analyzed
All the information used for calculation of nutritional parameters was obtained within one week before any treatment started. The following items were selected as concise constitutional evaluation methods: BMI = body weight (kg)/height (m)2 (underweight, <18.5 kg/m2), percentage of IBW = [height (cm)–80]×0.7/actual body weight (kg) for male or [height (cm)–70]×0.6/actual body weight (kg) for female(underweight, <90 per cent), Broca index defined as ideal weight (kg) defined = height (cm) −100 for male or = height (cm)–105 for female (underweight, actual body weight >Broca index), and PNI =10× albumin (g/dL) +0.005× total lymphocyte count (per mm3) (malnutrition, a PNI <50). A weight decrease more than 5% within three months was considered weight loss. Anemia was also considered as a nutritional item in our study (hemoglobin at least 12.0 g/dL for men and at least 11.0 g/dL for women).
Statistical analyses
All statistical analyses were performed by using Statistical Package for the Social Sciences (SPSS, Chicago, IL, version 13.0). The values were presented as the mean ± standard deviation for continuous data.
The Pearson’s χ2 test was used to compare the patient distribution between different subgroups. Kaplan-Meier method was used to estimate the 5-year overall survival (OS) and the log-rank test was used to determine the survival differences. OS time was calculated from the date of diagnosis to the date of death or last follow-up. For patients who remained alive, data were censored at the date of last contact. Cox proportional hazards model was used in the univariate and multivariate analyses for OS to determine the independent prognostic factors. A two-sided probability value of less than 0.05 was considered statistically significant.
Results
Patient and tumor demographics
A total of 502 ESCC patients were included, with a significant male predominance (female/male =0.31). The median age at initial diagnosis was 59 years, ranging from 20 to 90 years. The mid-thoracic esophageal cancer occurred most frequently (314/502, 62.5%) and most tumors had a well or moderate tumor grade (grade I-II, 334/502, 66.5%). More than half of the patients (286/502, 56.7%) presented with an advanced stage disease (stage III-IV) (Table 1).
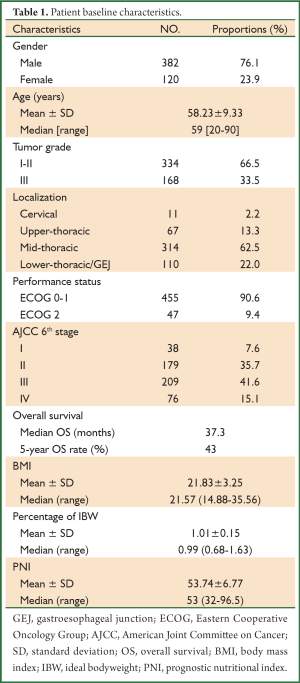
Full Table
Pretreatment nutritional status and OS
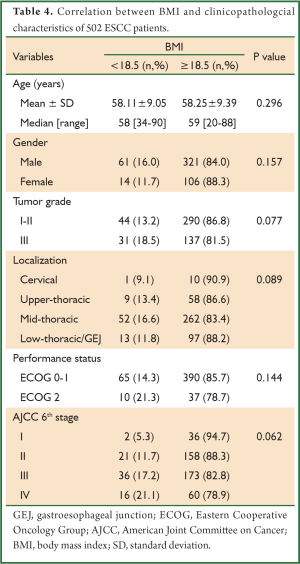
310 patients (61.8%) must be supplied with semi-fluid/fluid food or even could not orally take food, and 252 patients (50.2%) suffered from weight loss before diagnosis. The BMI, percentage IBW and PNI for the cohort were 21.83±3.25, 1.01±0.15 and 53.74±6.77 respectively. There were 75 patients (15.0%) considered as underweight, 344 patients (68.5%) as normal and 83 patients (16.5%) as overweight or obese by BMI. According to percentage of IBW, PNI and Broca Index, there were 127 (25.3%), 139 (27.7%) and 173 patients (34.6%) determined as underweight/malnutrition (Table 2).
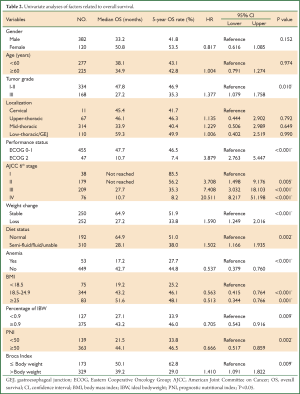
Full Table
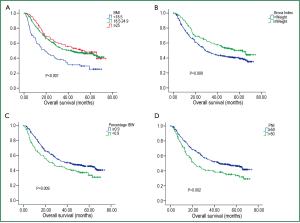
With a median follow up time of 30 months, the median OS for the entire patient group was 37.3 months with the 5-year OS rate of 43.0%. The median OS for patients with BMI less than 18.5, patients with BMI within 18.5-24.9 and patients with BMI more than 24.9 were 19.2, 43.2 and 51.6 months, respectively, with the 5-year OS rates of 25.2%, 46.1% and 48.1%. (P<0.001) (Figure 1A). Percentage of IBW, PNI, Broca Index and weight loss were also significantly associated with OS (P<0.01 for all) (Table 2, Figure 1B-D).
Univariate analyses and multivariate analyses
Univariate analyses using Cox regression model was performed to determine if age, gender, tumor grade, tumor localization, performance status, stage by AJCC 6th edition, dietary status, anemia or nutritional status (BMI, PNI, percentage IBW and Broca Index) were significantly associated with OS. Besides the indicators mentioned above for nutritional assessment, tumor grade, performance status, disease stage, dietary status and anemia were statistically significantly associated with OS. (P<0.05 for all) (Table 2).
Multivariate analyses for OS using Cox regression model were then performed to determine the independent prognostic factors, including all the items showing P values less than 0.05 in univariate analyses. Only performance status [unfavorable: ECOG 2; Hazard ratio (HR), 2.809; 95% confidence interval (CI), 1.962-4.020; P<0.001], AJCC 6th stage (unfavorable: stage III-IV; HR, 2.427; 95% CI, 1.846-3.191; P<0.001) and BMI (unfavorable: <18.5 kg/m2; HR, 1.693; 95% CI, 1.047-2.739; P=0.032) were the independent prognostic factors. Weight loss and a poorly differentiated type showed a tendency toward unfavorable survival, with P values (0.095 and 0.089, respectively) less than 0.10 (Table 3).
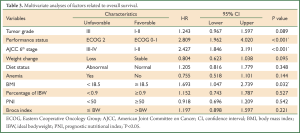
Full Table
Relationship between BMI and demographical information of ESCC patients
As BMI was an independent prognostic factor for ESCC patients, we further explored its correlation with clinicopathological characteristics of the entire cohort. As summarized in Table 4, patients with BMI <18.5 tended to present with a more advanced stage disease and a poorer differentiation grade, with P values close to 0.05 (Figure 2A,B). However, there were no differences across BMI groups (underweight or not) with respect to all the clinicopathological variables analyzed (P>0.05 across all groups).
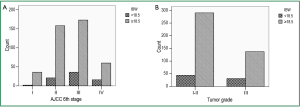
Full Table
Discussion
Our study comprehensively compared the prognostic values of various nutritional indictors and showed that pretreatment BMI was a useful predictor for ESCC patients’ outcome, independently from the other clinical and tumor characteristics. Meanwhile, BMI, as an indirect indicator for nutritional condition, performed more sensitively in predicting the clinical outcome of Chinese ESCC patients than other nutritional indicators did in the current study.
BMI is a steady nutritional indicator that is used worldwide, classifying the whole population into underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2) and obese (≥30 kg/m2) subgroups according to WHO criteria (20). Its prognostic impact on outcome has been already proposed and demonstrated in a series of cancers (7,8,21,22).
In esophageal cancer patients, BMI has previously been recognized as an epidemiological risk factor. A 23-year analysis of Norwegian cohort showed that BMI had opposite relations in the different histological groups of esophageal cancer, with low BMI increasing the risk of ESCC but high BMI decreasing the risk of esophageal adenocarcinoma (23). Another Chinese population-based prospective study of 22,000 men declared an inverse association between BMI and risk of esophageal cancer, while lower BMI correlated with increased esophageal cancer-related mortality (24). In the Western industrial world, the prevalence of overweight or obese population has increased rapidly over the past several decades as well as the incidence of esophageal adenocarcinoma. Thus, a great body of studies has been reported exploring the prognostic impact of high BMI in the esophageal cancer patients.
In 2009, Skipworth et al. conducted a retrospective study of 93 esophageal cancer patients undergoing surgical resection and found BMI (>25 vs. <25 kg/m2) as well as weight loss didn’t display reliably as an independent predictor of poor survival (25). Grotenhuis et al. reported their data from a retrospective study of 556 Netherlandish patients with esophageal cancer undergoing esophagectomy in 2010, showing that BMI class didn’t have prognostic value for short-term or long-term outcome (18). Hayashi et al. reported their experience from a cohort of patients undergoing surgery without adjuvant therapy in M.D. Anderson cancer center in 2010 and showed that high BMI (>25 kg/m2) was not an independent prognostic factor, which was in consistent with the results of the studies conducted by Melis et al. and Shridhar et al. (16,17,19) Another Netherlandish study of 736 esophagectomy patients by Blom et al. demonstrated a similar finding that a high BMI (>25 kg/m2) could not influence the 5-year OS rate (15). Wong et al. reviewed all the existing literatures analyzing the impact of elevated BMI on esophageal cancer as well as their own institutional outcomes from an esophageal cancer database, concluding that patients with high BMI didn’t necessarily correlate with increased postoperative complications or unfavorable outcome (20).
These studies in which BMI failed to display as a prognostic item had several limitations with regard to Asia population. Firstly, the BMI of the European or American patients enrolled was relatively higher compared with that of Eastern population, so that the prognostic impact of low BMI (<18.5 kg/m2) was rarely explored and not fully understood. Our cohort of Chinese patients presented with a lower mean BMI of 21.83±3.25 kg/m2, which was much lower than that reported by Western authors. Secondly, ESCC accounted for a small population (10-30%) and the relation between BMI and survival in ESCC was not explored. Finally, most of these studies included patients with early stage or fit enough who could afford a radical resection while patients with advanced stage or malnourished condition were rarely investigated.
Our study focusing on ESCC only demonstrated that a low BMI (<18.5 kg/m2) was an independent indicator for unfavorable OS in Chinese patients for the first time. In a large meta-analysis by Chinese oncologists (24), the death risk decreased 31% by an increased BMI of 5 kg/m2 for ESCC patients. A French study with a predominance of ESCC (87/105, 82.5%) declared a similar conclusion to our data (26). In that study, 105 patients (87 ESCC) with locally advanced esophageal cancer treated with definitive chemoradiation were retrospectively analyzed. Baseline nutritional status identified by a low albumin level (<35 g/L) and a BMI (<18 kg/m2) was associated with inferior survival in these patients. Clavier et al. analyzed 143 patients with advanced esophageal cancer (ESCC for 79%) from two French institutes and found a significantly prognostic impact of baseline nutritional status identified by nutritional risk index, a scoring system including BMI and albumin level (27).
The survival benefit for our ESCC patients with a relatively high BMI (≥18.5 kg/m2) might result from many aspects. A BMI less than 18.5 kg/m2 indicated the patient’s energy reservation was limited so that the tolerability for antitumor treatment might be relatively poor. Studies had found that nutritional intervention would decrease the incidence of postoperative complication, and hence indirectly supporting this hyposis (28,29). The treatment outcome of patients with inferior nutritional status was found to be unfavorable in case of undergoing definitive chemoradiation (26). Additionally, the poorer nutritional condition of ESCC patients identified by a low BMI, which was mostly caused by esophageal obstruction and active catabolism of inflammation mediators, would reflect the aggressiveness of the disease. Many authors demonstrated an inverse association between BMI and clinical stage at initial diagnosis (19,30). Similarly, our study found a tendency towards the negative association between TNM classification and BMI. Another interesting finding of our study was that a low BMI tended to be correlated with a poor differentiation grade of primary tumor.
Besides BMI, a series of other indicators also showed sensitively prognostic value in a couple of cancers, such as PNI for pancreatic cancer (12) and gastric cancer (10). These indicators were also explored in the current study. The survival difference between patient subgroups could be separated statistically in log-rank test by PNI, Broca Index, percentage IBW and weight loss. Unfortunately, the roles of these indicators as significantly independent predictor for OS were not identified in multivariate analysis, despite weight loss showed a tendency towards poor OS.
Our results are of interest in clinical practice. Baseline nutritional assessment using BMI on the basis of initial height and weight was objective and useful for predicting the clinical outcome of ESCC patients, independently from disease stage and performance status. Therefore, the baseline nutritional status should be carefully evaluated and it is helpful to the decision of individual treatment modality. For the ESCC patients with a low BMI (<18.5 kg/m2), we must give intensive nutritional support and improve the nutritional status in order to increase the tolerability of aggressive therapy, therefore to improve the prognosis. We are looking forward to future prospective studies taking the baseline nutritional condition and nutritional support into consideration.
The patients enrolled in our study presented with stages varying from early stage to metastatic stage, in a way could well reflect a real entity for ESCC in China. However, we found it was different to deeply investigate the prognostic impact of nutritional status on patients treated with different therapeutic modalities. That’s a disadvantage of our study.
Conclusions
In conclusion, our results suggest that the baseline nutritional status is predictive of OS in Chinese patients with ESCC. BMI is a steady indicator for nutritional evaluation and is a more sensitive prognostic parameter for ESCC patients, in contrast to other nutritional indicators. Furthermore, treatment optimization in ESCC patients with a low BMI should integrate the modalities and individual nutritional support.
Acknowledgments
We gratefully thank the staff members in the Department of Medical Oncology and Thoracic Surgery Oncology at Sun Yat-Sen University Cancer Center for their suggestion and assistance.
Disclosure: The authors declare no conflict of interest.
References
- Ott JJ, Ullrich A, Mascarenhas M, et al. Global cancer incidence and mortality caused by behavior and infection. J Public Health (Oxf) 2011;33:223-33. [PubMed]
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893-907. [PubMed]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Biggs K. Malnutrition screening programs in adult cancer patients: clinical practice is hungry for evidence. Curr Oncol 2012;19:e305-7. [PubMed]
- Santarpia L, Contaldo F, Pasanisi F. Nutritional screening and early treatment of malnutrition in cancer patients. J Cachexia Sarcopenia Muscle 2011;2:27-35. [PubMed]
- McRackan TR, Watkins JM, Herrin AE, et al. Effect of body mass index on chemoradiation outcomes in head and neck cancer. Laryngoscope 2008;118:1180-5. [PubMed]
- Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res 2008;14:1718-25. [PubMed]
- Migita K, Takayama T, Saeki K, et al. The Prognostic Nutritional Index Predicts Long-term Outcomes of Gastric Cancer Patients Independent of Tumor Stage. Ann Surg Oncol 2013;20:2647-54. [PubMed]
- Watanabe M, Iwatsuki M, Iwagami S, et al. Prognostic nutritional index predicts outcomes of gastrectomy in the elderly. World J Surg 2012;36:1632-9. [PubMed]
- Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today 2012;42:532-5. [PubMed]
- Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 2011;98:268-74. [PubMed]
- Riccardi D, Allen K. Nutritional Management of Patients With Esophageal and Esophagogastric Junction Cancer. Cancer Control 1999;6:64-72. [PubMed]
- Ligthart-Melis GC, Weijs PJ, Te Boveldt ND, et al. Dietician-delivered intensive nutritional support is associated with a decrease in severe postoperative complications after surgery in patients with esophageal cancer. Dis Esophagus 2013;26:587-93. [PubMed]
- Blom RL, Lagarde SM, Klinkenbijl JH, et al. A high body mass index in esophageal cancer patients does not influence postoperative outcome or long-term survival. Ann Surg Oncol 2012;19:766-71. [PubMed]
- Shridhar R, Hayman T, Hoffe SE, et al. Body mass index and survival in esophageal adenocarcinoma treated with chemoradiotherapy followed by esophagectomy. J Gastrointest Surg 2012;16:1296-302. [PubMed]
- Melis M, Weber JM, McLoughlin JM, et al. An elevated body mass index does not reduce survival after esophagectomy for cancer. Ann Surg Oncol 2011;18:824-31. [PubMed]
- Grotenhuis BA, Wijnhoven BP, Hötte GJ, et al. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J Surg 2010;34:2621-7. [PubMed]
- Hayashi Y, Correa AM, Hofstetter WL, et al. The influence of high body mass index on the prognosis of patients with esophageal cancer after surgery as primary therapy. Cancer 2010;116:5619-27. [PubMed]
- Wong JY, Shridhar R, Almhanna K, et al. The impact of body mass index on esophageal cancer. Cancer Control 2013;20:138-43. [PubMed]
- Park JM, Nam JS, Na W, et al. Prognostic value of body mass index in korean patients with castration-resistant prostate cancer. Korean J Urol 2012;53:761-5. [PubMed]
- Skírnisdóttir I, Sorbe B. Body mass index as a prognostic factor in epithelial ovarian cancer and correlation with clinico-pathological factors. Acta Obstet Gynecol Scand 2010;89:101-7. [PubMed]
- Engeland A, Tretli S, Bjørge T. Height and body mass index in relation to esophageal cancer; 23-year follow-up of two million Norwegian men and women. Cancer Causes Control 2004;15:837-43. [PubMed]
- Smith M, Zhou M, Whitlock G, et al. Esophageal cancer and body mass index: results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int J Cancer 2008;122:1604-10. [PubMed]
- Skipworth J, Foster J, Raptis D, et al. The effect of preoperative weight loss and body mass index on postoperative outcome in patients with esophagogastric carcinoma. Dis Esophagus 2009;22:559-63. [PubMed]
- Di Fiore F, Lecleire S, Pop D, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol 2007;102:2557-63. [PubMed]
- Clavier JB, Antoni D, Atlani D, et al. Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis Esophagus 2012. [Epub ahead of print]. [PubMed]
- Ligthart-Melis GC, Weijs PJ, Te Boveldt ND, et al. Dietician-delivered intensive nutritional support is associated with a decrease in severe postoperative complications after surgery in patients with esophageal cancer. Dis Esophagus 2013;26:587-93. [PubMed]
- Seike J, Tangoku A, Yuasa Y, et al. The effect of nutritional support on the immune function in the acute postoperative period after esophageal cancer surgery: total parenteral nutrition versus enteral nutrition. J Med Invest 2011;58:75-80. [PubMed]
- Hayashi Y, Correa AM, Hofstetter WL, et al. Patients with high body mass index tend to have lower stage of esophageal carcinoma at diagnosis. Dis Esophagus 2012;25:614-22. [PubMed]


