Indication for VATS sublobar resections in early lung cancer
Introduction
At present, surgery remains the most used radical treatment for early stage non-small cell lung cancer (NSCLC) (1). Lobectomy has been traditionally considered the gold standard procedure for early NSCLC following the Lung Cancer Study Group (LCSG) randomized controlled trial (2). However, the attempt to increase resection rates led to the need to offer surgery to patients with higher surgical risks: the elderly, the breathless and the ones with multiple co-morbidities (3-5). To manage these potential surgical risks and the possible long-term impairment in quality of life and respiratory function, surgeons have applied sublobar techniques to the management of lung cancer. These can be divided very clearly into two groups: non-anatomical resections (wedge) and anatomical resections (segmentectomies). The difference is the attempt during segmentectomies to follow the oncological principles of a lobectomy by achieving anatomical division of segmental veins, arteries and bronchi as well as good parenchymal clearance.
Video Assisted Thoracic Surgery (VATS) is on the increase in the management of benign and malignant processes. Large experiences have convinced the surgical community not only of the safety and possibilities of VATS surgery in early lung cancer, but of the benefits when compared to open surgery in terms of postoperative pain, length of recovery, return to activities, immune response to surgery and oncological results (6-9). As with open surgery where there is a variety of surgical approaches described (posterolateral, anterior, muscle-sparing, hybrid thoracotomies), VATS can also be performed with different surgical accesses: posterior approach, anterior approach, 2-port approach and single-port access (10-13).
We aimed to explore the potential possibilities and current experiences of the combination of sublobar resections and VATS techniques for early NSCLC.
Non-anatomical sublobar resections (wedge)
Wedge resections involve the excision of a pulmonary lesion with clear parenchymal margins with no attempt to deal with the hilar lobar structures (arteries, veins or bronchi). Although traditionally has been considered as a compromise operation due to the results of the LCSG trial that reported increase local recurrence compared to lobectomy, the indications for wedge excisions may be on the increase (2). Invariably, it is necessary that the lesion is peripheral so it can be identified and “wedged out” safely with sufficient margins. Despite the theoretical limitations as a sound oncologic procedure, wedge resection has continuously been used in certain circumstances for patients with lung cancer (14,15).
Technique
Wedge resections can be performed via VATS using a number of incisions including the single-port approach (16). Ideally the lung should be collapsed as it facilitates location of pulmonary nodules and instrumentation, but it can potentially be performed in a ventilated lung in patients that can’t tolerate single lung ventilation. There are different ways to identify the lesions including palpation with instruments or the tip of the finger, but also more complex techniques using technology such as placement of metal wires/coils (17,18), instillation of different contrasts (19-21) or use of intraoperative ultrasound techniques (22).
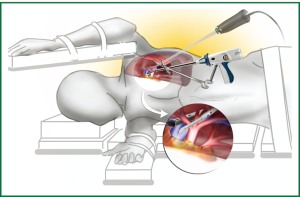
Once the nodule has been identified, surgical staplers are applied to excise and seal the pulmonary parenchyma with clear margins. A brief example of a diagnostic excision of a nodule in the left lower lobe via a single port incision is demonstrated in Video 1 with the position of the incision and instruments is illustrated in Figure 1.
Results
There is very limited evidence available to assess the role of wedge resections in lung cancer. One randomized controlled trial by the LCSG reported a similar survival, but increased recurrence of cancer in patients undergoing sublobar compared to lobar resections (2). The surgical community accepted the results and acknowledged the effort of the trialists and, even accepting the trial limitations, considered lobectomy as the procedure of choice for early lung cancer thus reserving sublobar resections for specific cohorts of patients who might benefit of the preservation of the parenchyma or a quicker procedure.
The experiences reported in the use of VATS wedge resections when compared to lobectomy are consistent with traditional reports in the thoracotomy approach. Wolf et al reported a retrospective comparative series of 154 sublobar resections (43% via VATS) and 84 lobectomies (10% via VATS) performed in patients with small early lung cancer. Patients who underwent lobectomy had a better survival and disease-free survival, but the sublobar group was significantly older and with worse respiratory reserve, highlighting the selection bias in this and every other study of its kind (23). Landreneau et al. reached similar conclusions in a multicenter study evaluating 102 wedge resections (60% by VATS) when compared to lobectomies (24).
One of the potential limitations of the use of VATS in deep-sited small lesions is the difficulty to locate them during surgery. The use of technologies has helped the identification of these nodules. Lee et al. were successful in 101 of 103 cases with small pulmonary nodules with the wire location techniques with an average operative time of 11 minutes (16). Molins et al. reported 50 out of 52 patients successfully underwent VATS excision of small nodules also identified by wires in the ambulatory setting (18). Similar success rates are reported by surgeons using different markers (methylene blue, radionuclides or contrast) (19-21). Finally, the use of intraoperative ultrasound has been reported by VATS, even in the single-port approach (25). Whatever the technology available, all these techniques seem to aid in identification of deep or small nodules during VATS surgery.
Indications
Based on the limited available evidence and the reported use of wedge resections in certain cohorts of patients with lung cancer we can identify possible indications for sublobar wedge resection in early NSCLC:
- Cases in which preservation of parenchyma is mandatory. These include patients with very limited pulmonary reserve with COPD, significant pulmonary fibrosis that carry poor prognosis when lobectomy is performed, pulmonary hypertension and, more recently, in the management of metachronous or synchronous lung cancers;
- Cases where preoperative histology could not be obtained or confirmed. Not only in very small pulmonary nodules unable to be biopsied percutaneously, but cases with history of distant malignancies where diagnosis metastasis/primary couldn’t be made, or when radiological appearances are not very suggestive of cancer but patients request histological confirmation;
- Diagnostic dilemmas in patients with underlying nodular lung disease (tuberculosis, sarcoid, rheumatoid) where one or more nodules are suspicious for malignancy during the course of their chronic disease in which a possible early NSCLC could be missed;
- Patients with severe comorbidities or very advanced age presenting with a peripheral nodule where a very short general anaesthesia period is preferred, where a wedge can be perform within few minutes, even with patients spontaneously ventilated.
Anatomical sublobar resections (segmentectomies)
Segmentectomies consist in the anatomical excision of one or more pulmonary segments. It is required to divide segmental branches of pulmonary artery, vein and bronchi related to the excised segments. The traditional technique of finding the segmental parenchymal plane by hand or electrocautery has now been substituted in many cases by the use of surgical staplers placed beyond the intersegmental plane with the potential benefit of reducing air leaks and parenchymal bleeding (26-28).
Segmentectomies for early lung cancer have been reported in the literature, and appear to be used more frequently (29,30). Surgeons have identified the potential role as an alternative to lobectomy in situations to increase operability (the elderly, patients with poor respiratory reserve, previous pulmonary resection) and resectability (multifocal ground-glass opacities, synchronous tumors, history of other solid malignancies where diagnosis of metastasis is a possibility), but also as the preferred option in small early stage NSCLC (31,32).
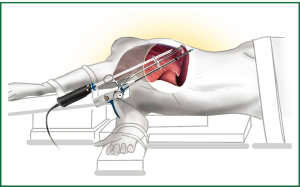
There is a limited but growing experience in the use of VATS segmentectomies, championed by experienced thoracoscopic surgeons but progressively being adopted by more units (33,34). The procedures can be performed via all the different VATS approaches including the Uniportal one (Video 2) and the instruments position is shown in Figure 2.
Technique
Segmentectomies can be divided into Typical (where parenchymal division involves 2 planes) or Atypical (more complex and technically demanding, when the segmental excision involves 3 planes). Examples of the former are excision of segments 6 on either side, lingulectomies, left apical upper tri-segmentectomies, left basal trisegmentectomies, right 7-10 segmentectomy. The rarer atypical segmentectomy examples are segmentectomy of 7-8 in the right, or 9-10 bilaterally.
With the patient on the lateral decubitus and forced hyperextension of the chest cavity to increase the intercostal space, a 4 cm incision is performed anterior to the latissimus dorsi edge at the level of 4th-5th intercostal space. The 30-degree thoracoscope is inserted to explore the pleural cavity. The thoracoscope is kept at the most posterior end of the wound allowing the insertion of 2, 3 or even more thoracoscopic instruments without interfering with them. Initially adhesions are divided with electrocautery and the left apical upper trisegmentectomy is performed. The Pulmonary Artery is identified and the initial branches are isolated and divided with an endo stapler. The segmental veins with preservation of the branches draining the lingula are then isolated and divided. Slightly more difficult is the identification of the segmental bronchus. Once this is isolated, we recommend that an inflation test is carried out prior to bronchial division as errors have been reported in VATS procedures. Once the bronchus has been divided, the parenchymal plane is identified by the inflation method prior to the excision. The specimen is removed with the help of a specimen bag in order to facilitate extraction and to minimize theoretical risk of wound seeding. A single intercostal drain is inserted after division of the inferior pulmonary ligament, lymph node excision and satisfactory lung re-expansion. Surgeons have employed other methods to identify the segmental plane: indocyanine green instillation or isolated inflation of the segments to be resected, all of them valid.
Results
The only randomized controlled trial including anatomical segmentectomies for lung cancer is the LCSG that, unfortunately, grouped segmentectomies together with wedge excisions. It concluded that survival after sublobar resections was equivalent to lobectomy but recurrence rates were much higher making a strong case for lobectomy to be considered the procedure of choice in early lung cancer. Unfortunately, the conclusions were impossible to extrapolate into a whole segmentectomy cohort due to the trial design (2).
Following this, few case-matched reports and several comparative series have indicated the value of anatomical segmentectomies to be similar to lobectomies in small size lung cancers, not only in the high-risk but also in the overall population (35-37). While survival or recurrence rates appear to be similar, there is evidence to demonstrate the lesser impact on pulmonary function after segmental resections.
If we apply the potential advantages seen in large experiences of surgeons performing VATS lobectomies compared with open lobectomies (less pain, early recovery, less complications and reduce immune response) the prospect of VATS anatomical segmentectomies might be very appealing (6-9). Several authors have described their experiences with a variety of VATS approaches from 4 to Single-port, and there are some comparative series between VATS and Open segmentectomy for lung cancer (38).
Overall, authors have not seen any significant differences in perioperative outcomes, survival or rates of recurrence between VATS segmentectomy and VATS lobectomy (Table 1) (39-43). The loco-regional recurrence rates vary between 2.8% and 7.7% in the different reports, similar to after VATS lobectomy by the same surgeons. One manuscript by Atkins et al compared the outcomes between open and VATS segmentectomies performed in an experienced thoracoscopic unit, with perioperative results indicating that VATS techniques do not compromise outcomes (38).

Full table
Authors have not seen a significant reduction in the patients’ hospital stay after VATS segmentectomy compared to VATS lobectomy, maybe as a consequence of longer lasting air leaks after segmentectomy due to the more extensive parenchymal trauma than after a fisureless VATS lobectomy (39-43). In the VATS experience we are yet to confirm the benefits on pulmonary function that segmentectomy seems to have over lobectomy in thoracotomy cohorts (44).
Indications
Based on the limited available evidence, and pending the results of modern studies underway (CALBG-140503 trial of segmentectomy vs. lobectomy for early lung cancer), the possible indications for VATS sublobar resections in NSCLC include:
- Nodules in patients with a previous history of solid malignancies in cases where intraoperative frozen sections can not differentiate a primary lung cancer from a distant metastasis;
- Multicentric ground glass opacities previously described as bronchoalveolar carcinoma;
- Second primary in cases who have undergone pulmonary resection in the past;
- Surgery in patients deemed to have a high-risk for a lobectomy including respiratory diseases, extreme age;
- An increasing number of segmentectomies are being used as procedure of choice in patients with peripheral early lung cancer of less than 2 cm.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Martin-Ucar AE, Waller DA, Atkins JL, et al. The beneficial effects of specialist thoracic surgery on the resection rate for non-small-cell lung cancer. Lung Cancer 2004;46:227-32. [PubMed]
- Riaz SP, Linklater KM, Page R, et al. Recent trends in resection rates among non-small cell lung cancer patients in England. Thorax 2012;67:811-4. [PubMed]
- Griffin JP, Eastridge CE, Tolley EA, et al. Wedge resection for non-small cell lung cancer in patients with pulmonary insufficiency: prospective ten-year survival. J Thorac Oncol 2006;1:960-4. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [PubMed]
- Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. [PubMed]
- Walker WS, Carnochan FM, Pugh GC. Thoracoscopic pulmonary lobectomy. Early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg 1993;106:1111-7. [PubMed]
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [PubMed]
- Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg 2007;133:325-32. [PubMed]
- Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010;90:875-81; discussion 881-3. [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [PubMed]
- Li W, Wang Y, He X, et al. Combination of CT-guided hookwire localization and video-assisted thoracoscopic surgery for pulmonary nodular lesions: analysis of 103 patients. Oncol Lett 2012;4:824-8. [PubMed]
- Molins L, Mauri E, Sánchez M, et al. Locating pulmonary nodules with a computed axial tomography-guided harpoon prior to videothoracoscopic resection. Experience with 52 cases. Cir Esp 2013;91:184-8. [PubMed]
- Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labeling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg 1998;14:265-70. [PubMed]
- Choi BG, Kim HH, Kim BS, et al. Pulmonary nodules: CT-guided contrast material localization for thoracoscopic resection. Radiology 1998;208:399-401. [PubMed]
- Stiles BM, Altes TA, Jones DR, et al. Clinical experience with radiotracer-guided thoracoscopic biopsy of small, indeterminate lung nodules. Ann Thorac Surg 2006;82:1191-6; discussion 1196-7. [PubMed]
- Piolanti M, Coppola F, Papa S, et al. Ultrasonographic localization of occult pulmonary nodules during video-assisted thoracic surgery. Eur Radiol 2003;13:2358-64. [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 1824-5.
- Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691-8; discussion 698-700. [PubMed]
- Rocco G, Cicalese M, La Manna C, et al. Ultrasonographic identification of peripheral pulmonary nodules through uniportal video-assisted thoracic surgery. Ann Thorac Surg 2011;92:1099-101. [PubMed]
- Jones DR, Stiles BM, Denlinger CE, et al. Pulmonary segmentectomy: results and complications. Ann Thorac Surg 2003;76:343-8; discussion 348-9. [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [PubMed]
- D’Amico TA. Thoracoscopic segmentectomy: technical considerations and outcomes. Ann Thorac Surg 2008;85:S716-8. [PubMed]
- Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer. A fifteen-year experience. J Thorac Cardiovasc Surg 1973;66:563-72. [PubMed]
- Yang CF, D’Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [PubMed]
- Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient--the benefits of avoiding the gold standard. Eur J Cardiothorac Surg 2010;38:6-13. [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1.
- Shiraishi T, Shirakusa T, Iwasaki A, et al. Video-assisted thoracoscopic surgery (VATS) segmentectomy for small peripheral lung cancer tumors: intermediate results. Surg Endosc 2004;18:1657-62. [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [PubMed]
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [PubMed]
- Date H, Andou A, Shimizu N. The value of limited resection for “clinical” stage I peripheral non-small cell lung cancer in poor-risk patients: comparison of limited resection and lobectomy by a computer-assisted matched study. Tumori 1994;80:422-6. [PubMed]
- Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 961. [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. [PubMed]
- Shapiro M, Weiser TS, Wisnivesky JP, et al. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg 2009;137:1388-93. [PubMed]
- Yamashita S, Chujo M, Kawano Y, et al. Clinical impact of segmentectomy compared with lobectomy under complete video-assisted thoracic surgery in the treatment of stage I non-small cell lung cancer. J Surg Res 2011;166:46-51. [PubMed]
- Soukiasian HJ, Hong E, McKenna RJ Jr. Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J Thorac Cardiovasc Surg 2012;144:S23-6. [PubMed]
- Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. [PubMed]
- Zhao X, Qian L, Luo Q, et al. Segmentectomy as a safe and equally effective surgical option under complete video-assisted thoracic surgery for patients of stage I non-small cell lung cancer. J Cardiothorac Surg 2013;8:116. [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [PubMed]