
Complete video-assisted thoracoscopic surgery upper left bronchial sleeve lobectomy
Introduction
Video-assisted thoracoscopic surgery (VATS) lobectomy has been proven as a minimally invasive, safe, and feasible surgical approach for early-stage non-small cell lung cancer (NSCLC) (1,2). However, the application of complete VATS bronchial sleeve lobectomy has been somewhat limited due to a complicated and challenging process for bronchial anastomosis. Sleeve resection used to be viewed as an absolute contraindication to VATS lobectomy, and few studies were reported (3,4). Here, we reported a case undergoing complete VATS upper left bronchial sleeve lobectomy.
Operative techniques (Video 1)
The patient was placed in a 90-degree side lying position on the unaffected side under double-lumen endotracheal anesthesia. The operator stood facing the abdomen. A 1.5-cm observation port was created in the 7th intercostal space at the ipsilateral middle axillary line, a 3-cm working port in the 4th intercostal space at the ipsilateral anterior axillary line, and a 2-cm auxiliary port in the 7th intercostal space at the ipsilateral posterior axillary line. An incision protection retractor was attached to each port. The inferior pulmonary ligament was initially divided to expose the mediastinal pleura.
Typically, single-direction lobectomy could be considered for the procedure. In view of her poorly developed oblique fissure, however, bronchial sleeve lobectomy was required for the central type lung cancer in this patient. Therefore, we planned to divide the oblique fissure first, dissect the left upper pulmonary vein and the upper lobe branch of the left pulmonary artery, and eventually perform the sleeve resection.
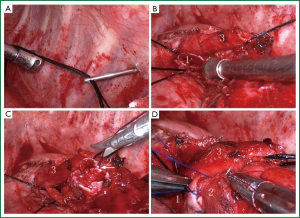
The left upper pulmonary vein was divided. The oblique fissure was partially dissected with an electrotome, and the hypoplastic interlobular fissures were treated with a linear stapler. With the oblique fissure exposed, the upper lobe branch of the left pulmonary artery was divided, and the lingular segment of the left pulmonary artery, the left upper pulmonary vein, and the proper segment of the left pulmonary artery were treated with a linear stapler in this order. The surrounding connective tissue of the left main bronchus and the upper and lower lobe bronchi of the left lung was removed. The left main bronchus and the left lower bronchus were dissected near the orifice of the left upper bronchus using scissors and a scalpel. Frozen sections of the cut ends of the left main bronchus and the dorsal and basal segments of the left lower lung were negative of tumor infiltration as confirmed pathologically during surgery. Stations 4, 5, 6, 7, 9, 10, 11 and 12 lymph nodes were dissected. Left bronchial sleeve lobectomy was then performed. For better exposure and accessibility, we raised the left main bronchus by passing two 1-0 silk sutures, respectively ligated with both sides of the wall of the left main bronchus (Figure 1), through the anterior and posterior chest walls using a crochet needle, and lifted the trunk of the left pulmonary artery by threading a 1-0 silk suture through the posterior chest wall with a crochet needle. In this case, since the cut end of the bronchus was so close to the left lower bronchus that its dorsal and basal segments were separated, we reconstructed the carina between the two using a 4-0 absorbable suture (Figure 1). The left main and left lower bronchi were then joined together using a single continuous suture (Figure 1). Leak testing was conducted following the anastomosis, in which no leakage was detected up to an airway pressure of 30 cm H2O (2.94 kPa).
Discussion
A full-thickness interrupted suture was used in bronchial anastomoses in the published studies (5,6), while Liu et al. reported the use of a continuous suture (7). We modified the latter method in this case. We used 3-0 or 4-0 prolene sutures in bronchial anastomosis-with the first suture made in the inner side of the bronchial wall and knotted outside the bronchus, a continuous suture was made on one quarter of the circumference on both sides and drawn to reduce the tension, followed by further continuous sutures to cover the entire circumference, with a final knot at the outer side of the wall to complete the anastomosis. The tension of each suture was adjusted before knotting to avoid slack. With respect to the need for intraoperative autologous tissue coverage during the anastomosis, Mahtabifard et al. have suggested that no evidence is available for the supportive effect of autologous tissue in bronchial anastomoses (6). Despite lack of autologous tissue coverage in our case, intraoperative and postoperative bronchoscopy revealed no sign of anastomotic stenosis or anastomotic leakage, and the outcome of the anastomosis was considered satisfying.
We noted that, during the bronchial anastomosis in this case, sutures were prone to mingle and confuse with each other. As reported by Liu et al. (5), in order to prevent such occurrence during a full-thickness interrupted suture for bronchial anastomoses, each suture was closed with a mosquito clamp as a marker, and placed on oval forceps in order to avoid confusion. We closed bronchial anastomosis using a single continuous prolene suture, and sent those sutures that were not in use during the process out from the auxiliary port, which greatly reduced the presence and entanglement of sutures, as well as the operation time.
Mahtabifard et al. has reported the sleeve reconstruction of all lobe bronchi except that in the left upper lobe (6). The deep location of the operative field partly hidden under the left pulmonary artery trunk during bronchial anastomosis in this region has made it even more difficult to operate thoracoscopically. To improve exposure of the operative field, we managed to raise the left main bronchus by passing two 1-0 silk sutures, respectively ligated with both sides of the posterior wall of the left main bronchus, through the anterior and posterior chest walls using a crochet needle. Similarly, a 1-0 silk suture was advanced through the posterior chest wall with a crochet needle to lift the trunk of the left pulmonary artery. In this way, a widely open, exposed field was achieved.
In conclusion, complete VATS bronchial sleeve lobectomy is a minimally invasive approach for thorough removal of tumor lesions while sparing as most normal lung tissue as possible, which avoids pneumonectomy and satisfies the psychological and physiological needs of patients. With the extensive application of complete VATS lobectomy, techniques for bronchial sleeve lobectomy have been increasingly reported, and the key to successful performance is the end-to-end anastomosis under thoracoscope.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Predina JD, Kunkala M, Aliperti LA, et al. Sleeve lobectomy: current indications and future directions. Ann Thorac Cardiovasc Surg 2010;16:310-8. [PubMed]
- Li Y, Wang J. Video-assisted thoracoscopic surgery sleeve lobectomy with bronchoplasty. World J Surg 2013;37:1661-5. [PubMed]
- Liu HC, Zhu YM, Jiang GN, et al. Video-assisted thoracoscopic surgery rupper upper bronchial sleeve lobectomy: report of two cases. Chin J Clin Thorac Cardiovasc Surg (Chin) 2011;10:10.
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [PubMed]
- Liu LX, Mei JD. Video-assisted thoracoscopic surgery bronchial sleeve lobectomy for lung cancer: report of preliminary experience. Chin J Clin Thorac Cardiovasc Surg (Chin) 2011;10:387-9.


