LncRNA260-specific siRNA targeting IL28RA gene inhibit cardiomyocytes hypoxic/reoxygenation injury
Introduction
A more complete understanding of cardiomyocyte apoptosis holds great therapeutic potential to the medical community due to the phenomena’s prominent role in a variety of cardiac diseases, including acute myocardial infarction (AMI) rat, heart failure, and cardiac allograft (1). In the search for a mechanism, IFN-λs (also known as type III interferons) are of particular interest due to their demonstrated effect on cell proliferation and apoptosis in both oncology and cardiology. The functional receptor for these interferons consists of a heterodimer formed by interleukin 28 receptor alpha (IFN-λ-R1/IL-28Rα, IL28RA, CRF2-12) and interleukin 10 receptor beta (CRF2-4, IL10-R-β, ILl0RB).
Given the similar signal transducer pathway between type I IFNs and IFN-λs, Dumoutier et al. speculated IFN-λs may inhibit angiogenesis and restrain tumor growth in vivo (2), a phenomenon that is now well-documented (3). IFN-λ blocks cell proliferation by binding with the IL28RA/ILl0RB cytokine receptor complex, which then inhibits the JAK-STAT signaling pathway and the downstream PI3K/AKT and MAPK signaling pathways. On the other hand, decreasing expression of IL28RA, a component of the IFN-λ receptor complex, could have the opposite effect of promoting cell proliferation and inhibiting apoptosis by increasing signaling through the JAK-STAT and PI3K/AKT pathways. This idea confirmed in 2010 by a meta-analysis performed by Yang et al. who found that, in certain cancers, decreased levels of IL28RA were closely associated faster tumor growth and increased patient mortality (P=0.014) (3,4). The same property that makes these cancers so lethal, however, holds promise for patients in the cardiac setting (3). Performing semi-quantitative PCR on mouse myocardium damaged by chlorpromazine (CPZ), Tsai et al found increased levels of IL28RA and decreased levels of the anti-apoptotic gene Bcl-2, leading them to speculate that the IL28RA gene plays an important role in facilitating the cardiomyocytes apoptosis that leads to myocardial damage and mortality (5).
Recently, some studies have suggested long non-coding RNAs (lncRNAs) in cardiovascular physiology and the initiation and progression of cardiovascular disease (6-8). Early on in our study, we tested what genes were regulated by lncRNA260 by high throughput RNA and lncRNA chips screening and found that only the IL28RA gene was the only gene to be regulated by lncRNA260. Based on these findings, we hypothesized that lncRNA260-specific siRNA-mediated inhibition of IL28RA would protect cardiomyocytes from hypoxic reoxygenation (H/R) injury and indeed, all three different forms of siRNA induced increased cell survival and decreased levels of apoptosis in cardiomyocytes after H/R (9). We also confirmed the activation of the JAK-STAT and PI3K/AKT signaling pathways and identified phosphatidylinositol 3-kinase p110 gamma (PI3KCG) as the primary downstream gene regulated by IL28RA gene.
Methods
Materials
LipofectamineTM2000 (Invitrogen, CA, USA), collagenase and Dulbecco Modified Eagle medium (DMEM) cell culture medium (Gibco), lactate dehydrogenase (LDH) and creatine kinase (CK) detection kit (Nanjing Jiancheng Biology Limited Company, Nanjing, China), cell counting kit-8 (CCK-8) cell proliferation and toxicity detection kit (Dojindo), apoptosis and necrosis assay kits (Cat C1056) (Beyotime Biotechnology), IL28RA rabbit monoclonal antibody (Sigma). PI3KCG mouse antibody (Santa Cruz), β-actin rabbit antibody (Cell Signaling), Bcl-2 rabbit antibody (Cell Signaling), Bax rabbit antibody (Cell Signaling), rabbit anti-rat caspase 3 antibody (Cell Signaling), phospho-AKT (pAKT) (Ser 473) rabbit antibody (Cell Signaling), Donkey F(ab)2 Anti-Rabbit IgG H&L (Alexa Fluor® 568) preadsorbed (Abcam).
Purebred healthy Sprague-Dawley (SD) rats less than 3 days old were provided by the Jiangsu Province Animal Center. Neonatal SD rats were between 6 and 8 grams. Three pairs of lncRNA260-specific siRNA and negative control hydroxyl fluorescein (FAM-siRNA) were designed and synthesized by Shanghai Gene Pharma Co., Ltd. The nucleotide sequences are listed in Table 1.
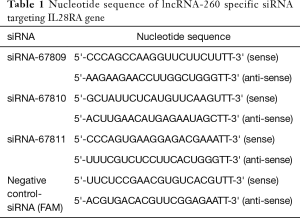
Full table
Cardiomyocytes isolation and culture (10)
The current research was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2009-SR-033.1). The ventricular tissue was minced then moved to 10 mL of 0.1% collagenase solution to digest the cardiac muscle tissue. After 8 min of digestion on the shaking table at 37 °C repeating for three times, the solution was centrifuged for 10 minutes at 1,000 rpm at 4 °C. After removing the supernatant, the collected cells were resuspended in 8 mL DMEM supplemented with 10% fetal bovine serum (FBS) and inoculated in a 10-cm diameter culture dish. After incubating the cells at 37 °C under 5% CO2 flow for 90 minutes, dishes were aspirated and cells transferred to 96-well or 6-well plates at a density of 1×105 cells/mL. After 72 hours, cardiomyocytes were randomly divided into six groups: normal control group, H/R group, H/R group + negative control transfection group, H/R + siRNA-67809 transfection group, H/R + siRNA-67810 transfection group, and H/R + siRNA-67811 transfection group.
Transfection of cardiomyocytes with siRNA
FAM is a green fluorophore with an excitation wavelength of 480 nm and a launch wavelength of 520 nm. We found that 100 nmol/L FAM-siRNA to be the appropriate concentration for transfecting primary neonatal rat cardiomyocytes.
After culturing primary cardiomyocytes for 3 days, penicillin/streptomycin was removed from the culture medium and siRNA-67809, siRNA-67810, and siRNA-67811 were transfected into the cardiomyocytes at the above concentration. Triplicate wells were set up in each group. After the lipofectamine 2000 dissolved in DMEM medium was mixed with siRNA, the lipofectamine-siRNA mixture was added into the cardiomyocytes cell culture plate wells respectively and gently mixed. After transfection for 6 hours, the transfection efficiency was measured under the fluorescence microscope and the lipofectamine 2000, removed. The culture medium was changed with new complete DMEM medium with 10% FBS and penicillin/streptomycin.
Establishing the in vitro cardiomyocyte H/R model (11)
72 hours after cardiomyocytes transfection, all groups except controls were treated with hypoxia for 6 hours using Anaerobic bag (Becton Dickinsonand Inc., USA) (95% N2 and 5% CO2) and then normoxia for 2 hours (74% N2, 21% O2, and 5% CO2) to simulate the H/R process.
Cardiomyocytes beat frequency detection
After the H/R treatment, three views were randomly selected from each well under an inverted microscope at room temperature. After measuring the cardiomyocyte beat frequency in beats per minute, the average value between each set of three parallel wells was calculated. The seeding density in each group is 1×105 cells/mL. The beat frequency calculations have been normalized according to the same seeding density.
Measuring cell viability detection by CCK-8
After addition of 10 µL CCK-8 solution into each well, plates were incubated for one hour. Absorbance was then measured using a microplate reader at 450 nm. The cell viability percentage (%) = [(the absorbance value in the experiment group − the absorbance value in the blank control group) / (the absorbance value in the normal culture group − the absorbance value in the blank control group)] × 100%. All samples were discarded after measuring. Control wells, only consisting of a culture medium and CCK-8 solution, contained no cells.
Measuring LDH or CK activity in the cell culture medium supernatant
LDH or CK activity was measured using the LDH or CK detection kit. For LDH measurement, 20 µL cell culture medium supernatant from each group was transferred to a microplate and absorbance was subsequently measured using a microplate reader at 450 nm. LDH activity (U/L) = [(the experiment OD value − the control OD value) / (the standard OD value − the blank control OD value)] × standard substance concentration (0.2 mmol/L) × 1,000. The blank control was not consisted of any sample or enzyme-labelled regents.
According to the CK activity detection kit, the OD value was measured with a microplate reader and 1cm optical path at 660 nm. CK activity (U/mL) = [7.4491 × (the experiment OD value − the control OD value) − 0.0716] × sample dilution times before measurement.
Measuring rate of cardiomyocyte apoptosis
The cardiomyocytes apoptosis rates were assayed by using the Hoechst 33342 and propidium iodide (PI) double staining methods. The 5 uL Hoechst 33342 was added into each well of the 6-well plates. Then 5 µL PI was then added into each well. Hoechst 33342 and PI were mixed and incubated with the cardiomyocytes on ice for 30 min. The cardiomyocytes were then washed once with PBS before being observed under the inverted fluorescent microscope.
Measuring protein expression of cultured cardiomyocytes by Western Blot
Total protein was extracted from the cultured cardiomyocytes using RIPA lysis buffer. After protein quantification, 36 µg of total protein was separated on a 10% SDS-PAGE gel and subsequently transferred to polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was blocked with 5% skim milk powder at 4 °C overnight. The 1:1,000 diluted primary antibodies (rabbit anti-rat β-actin antibody, rabbit anti-rat IL28RA antibody, mouse anti-rat PI3KCG antibody, rabbit anti-rat pAKT antibody, rabbit anti-rat Bcl-2 antibody, rabbit anti-rat Bax antibody) were incubated with the proteins at 4 °C overnight. PI3KCG was the key protein in the active PI3K/AKT downstream pathway which was supposed to be elevated after the three pairs of siRNAs were transfected into the cardiomyocytes. Bax was the apoptosis promoting protein and Bcl-2 was the apoptosis inhibiting protein. The 1:5,000 diluted horseradish peroxidase (HRP) labeled secondary antibody (goat anti rabbit IgG antibody, goat anti mouse IgG antibody) was added and incubated with the proteins for 2 hours at 4 °C. The protein transferred and antibodies incubated PVDF membrane was developed with Thermo scientific pierce Super Signal West Femto Chemiluminescent Substrate for 1 min. After washing the PVDF membrane in double-distilled water, it was observed under the Gel Imaging System. Western blots results were photographed and recorded. The proteins optical density (OD) was calculated.
Measuring IL28RA and caspase 3 proteins expression of cultured cardiomyocytes by immunofluorescence staining
After the H/R process, the cardiomyocytes growing on the glass slide in 24-well plates were then rinsed using phosphate buffer saline (PBS) and fixed in 4% paraformaldehyde for 15 min at room temperature (RT). After rinsing cardiomyocytes in PBS for 3 times, the cardiomyocytes were permeabilized with 0.2% Triton X 100 for 5 min at room temperature for caspase 3 which would transfer to the nucleus during the apoptosis process. For IL28RA protein, it does not need the permeabilization because it is distributed in the cytoplasm and cytomembrane. Then the cardiomyoctes were blocked with normal lamb serum (3% in PBS) for 45 minutes at RT after they were rinsed with PBS for 3 times. Then the cardiomyoctes were incubated with IL28RA or caspase 3 rabbit anti-rat polyclonal antibodies at a 1:100 dilution at 4 °C overnight respectively. Afterwards, the cardiomyoctes were rinsed with PBS and then incubated for 1 h with a 1:1,000 dilution of Alexa Fluor® 568-conjugated donkey anti-rabbit secondary antibodies at room temperature in a lucifugal humidified box for 1 h. Then 4’, 6-diamidino-2-phenylindole (DAPI) was added to the cardiomyoctes for 5 minutes after washing them with PBS. Then the glass slide was picked out and mounted with Anti-Fade Fluoresence Mounting Medium. The cardiomyoctes on the glass slide were observed with fluorescent microscope and photographed.
Statistical analysis
The data were analyzed by using SPSS 20.0 statistical software. Measurement data were used as mean ± standard deviation in the name of (
Results
The beat frequency and survival rates compared in the cardiomyocytes H/R process
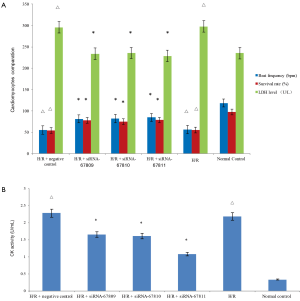
While unattached cardiomyocytes present as circular holes, they gradually became fusiformis, polygonal as they attach. Cells fused to the island began beating on the third day. While the H/R group experienced a significantly decreased beat frequency and survival rate compared to controls (P<0.05), the three groups transfected with the lncRNA260-specific siRNA experienced increased cell viability. (P<0.05) (Figure 1A).
The LDH and CK level comparison in the cardiomyocytes H/R process
The supernatant of the H/R group and H/R+ negative transfection groups had significantly greater amounts of LDH and CK levels than that of the controls (P<0.05). The supernatant of the three siRNA groups, on the other hand, however, contained expressed significantly decreased levels of LDH and CK (P<0.05) (Figure 1A,B).
Cardiomyocytes apoptosis assay comparison results in the H/R process
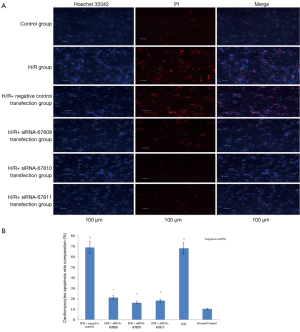
After being double stained by Hoechst 33342 and PI, cardiomyocytes that did not undergo H/R treatment presented a uniformly dispersed blue fluorescence with an apoptosis rate of only 10.2%±0.1%. Cardiomyocytes in the H/R and H/R + negative transfection groups, however, were in the mid to late apoptosis stage, presented a non- uniform fluorescence, and were highly concentrated with strong blue and red fluorescence. The apoptosis rate in these groups were 67.9%±2.5%, 68.7%±2.5% respectively (P<0.01). siRNA transfection decreased the apoptosis rates to only 21.1%±1.2%, 16.1%±1.3%, and 18.3%±1.1%, respectively (P<0.01). Compared with the H/R and H/R+ negative transfection groups, the strong blue and red flurescence signals were significantly decreased in the three siRNA transfection groups (Figure 2A,B).
Protein expression detection by western blot in the cardiomyocytes H/R process
Although H/R and H/R + negative control groups had increased expression of IL28RA and decreased expression PI3KCG relative to the transfection group, both groups had decreased IL28RA and PI3KCG when compared to the normal control group (Figure 3A). This suggests that siRNA works by inhibiting IL28RA while promoting PI3KCG.
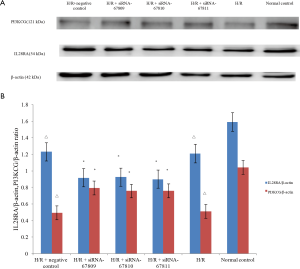
Next, we looked at the ratio between PI3KCG/β-actin protein expressions. We found no significant difference between the H/R and H/R + negative control groups (P>0.05) and both groups had decreased PI3KCG/β-actin ratios relative to controls (Figure 3B) (0.49±0.003, 0.51±0.005, respectively, P<0.05; compared to control: 1.04±0.006). The siRNA transfection group had a higher PI3KCG/β-actin ratio when compared to that of the H/R and H/R + negative transfection groups (0.79±0.004, 0.76±0.003, 0.76±0.005, respectively, P<0.05).
In our evaluation of the ratio of IL28RA/β-actin, we again observed no significant difference between the H/R group and H/R + negative control group (P>0.05) and that values for these two groups were lower compared to that of controls (1.23±0.003, 1.21±0.005, respectively, P<0.05; compared to control: 1.59±0.006). On the other hand, the IL28RA/β-actin ratio of the siRNA transfection groups were lower than that of the H/R and H/R + negative control groups (0.92±0.004, 0.92±0.003, 0.90±0.005, respectively, P<0.05).
Then, we observed the pAKT/β-actin protein expressions ratio in Figure 4. The H/R and H/R + negative control groups had increased pAKT/β-actin ratios relative to controls (0.390±0.002, 0.399±0.003, respectively, P<0.05; compared to control: 0.243±0.006). The siRNA transfection group had a significantly higher pAKT/β-actin ratio when compared to that of the H/R and H/R + negative transfection groups (0.627±0.004, 0.648±0.003, 0.656±0.005, respectively, P<0.05) which suggested that the siRNAs promote the AKT phosphorylation and active the PI3K/AKT signal pathway (Figure 4A,B).

Our results for levels of Bax and Bcl-2 proteins were concordant with our results from the cardiomyocyte apoptosis assay. The siRNA transfected groups expressed lower levels of Bax and higher levels of Bcl-2 protein compared to the H/R and H/R + negative control group (P<0.05). Compared with the normal control group, the Bax and Bcl-2 protein in the H/R group and H/R + negative transfection group were significantly increased (P<0.05) (Figure 5A). This suggested that the siRNA had an inhibitory effect on the Bax protein expression while promoting Bcl-2 protein expression, confirming the lnc260-specific siRNA’s ability to both inhibit apoptosis and promote survival.
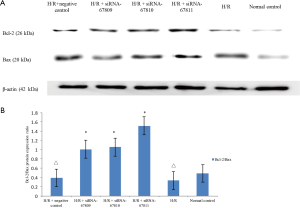
As we see in Figure 5B, the Bcl-2/Bax ratio decreased in H/R and H/R + negative controls compared to the control group (0.331±0.005, 0.392±0.003, respectively, P<0.05, compared to normal control: 0.486±0.006). Compared with H/R and H/R + negative transfection groups, the three siRNA transfection groups expressed a higher Bcl-2/Bax ratio (1.009±0.004, 1.055±0.003, 1.514±0.005, respectively, P<0.05).
No significant difference was detected in Bcl-2/Bax ratio between H/R and H/R + negative transfection groups (P>0.05).
The IL28RA, cleaved caspase 3 proteins localization and relative levels by immunostaining in the cardiomyocytes H/R process
As we see in Figure 6A, the IL28RA protein is localized in the cytoplasm and cytomembrane, not in the nucleus. The IL28RA fluorescence intensity is significantly decreased in the H/R group and H/R + negative control group, compared with the normal control group (0.78±0.003, 0.77±0.004, respectively, P<0.05; compared to control: 1.00). After the three siRNAs were transfected into the cardiomyocytes, the IL28RA fluorescence intensity is further decreased which suggested that the lncRNA 260 siRNAs inhibit the IL28RA expression (0.60±0.002, 0.61±0.003, 0.62±0.004, respectively, P<0.05). The relative value of IL28RA fluorescence intensity was shown in Figure 6B.

As Figure 7A has shown that the cleaved caspase 3 is localized in the nucleus. The cleaved caspase 3 fluorescence intensity is significantly increased in the H/R group and H/R + negative control group, compared with the normal control group (1.60±0.08, 1.65±0.075, respectively, P<0.05; compared to control: 1.00). After the three siRNAs were transfected into the cardiomyocytes, the cleaved caspase 3 fluorescence intensity is decreased which suggested that the lncRNA 260 siRNAs inhibit the cleaved caspase 3 expression (1.22±0.025, 1.19±0.030, 1.23±0.028, respectively, P<0.05). The relative value of cleaved caspase 3 fluorescence intensity was shown in Figure 7B.

Discussion
Cardiac ischemia reperfusion (I/R) injury is an unavoidable aspect of the current standard of the treatment for AMI. After treatment by thrombolysis and percutaneous transluminal coronary angioplasty therapy, the ensuing cardiomyocytes apoptosis and necrosis, a hallmark of cardiac I/R injury, often leads to AMI aggravation. Inhibiting cardiomyocytes apoptosis, therefore, is essential to treating AMI and also congestive heart failure (12).
To test whether inhibiting IL28RA could protect cardiomyocytes from H/R injury, we transfected cardiomyocytes with three pairs of siRNA (siRNA-67809, siRNA-67810 and siRNA-67811) all designed to interfere with lncRNA260, a key regulator for the IL28RA gene. RNA interference (RNAi) is a common biological phenomenon, where siRNA binding with its complementary messenger RNA (mRNA) results in the degradation of the mRNA molecule, thus inhibiting its translation. RNAi-mediated gene expression is easier than conventional gene knockouts approaches and also possesses a high specificity and efficiency compared with antisense oligonucleotides, RNA enzymes, and DNA enzymes (13-16).
IFN-λs and their associated cytokine receptor IL28RA/ILl0RB exist throughout the human body in various kinds of tissues (3), but while the IL10RB serves as a part of the receptors of IL10, IL22 and IL26 (17), the IL28RA chain is specific to IFN-λ’s, making it the ideal mechanistic component. The IL28RA gene is located in 1p36.11, close to the gene encoding IL22 receptor. We first tested the viability of our cardiomyocyte H/R model by comparing cell viability and LDH activity levels between the H/R treatment group and control group. As expected, the experimental group showed about 50% decreased cardiomyocyte viability, 2 folds increased LDH activity levels and 7 folds increased CK activity levels.
We then wanted to test the viability of the three pairs of lnc260-specfic siRNA. We transfected three groups of cardiomyocytes with the three pairs of siRNA and then subjected each group to H/R treatment. After measuring the beat frequency and overall survival, we found that all three siRNA-transfected groups exhibited about 1.5 folds higher beat frequencies and survival rates than the H/R and the H/R + vehicle groups. The three siRNA-transfected groups also exhibited about 25% lower levels of LDH, 30–50% lower levels of CK, and a 60% decreased rate of apoptosis (P<0.05). Thus, we determined all three pairs of siRNA were capable of optimally protecting cardiomyocytes through the inhibition of IL28RA expression.
PI3K/AKT was an important intracellular signal transduction pathway that regulates cell proliferation, differentiation, glucose metabolism and migration (18). It also indirectly regulates calcium channels to influence myocardial contractility mediates cell growth and proliferation, protein synthesis, and apoptosis (19-21). To learn more about the mechanism responsible for our results, we used Western blots to measure differences in protein expression and found that treatment with the three siRNAs decreased expression of IL28RA and the apoptosis promoter Bax and cleaved caspase 3 while increasing expression of PI3KCG, pAKT and Bcl-2, an apoptosis inhibitor. This confirmed that lncRNA260 promoted IL28RA transcription and that its interference by siRNA effectively reduced IL28RA gene expression and activated the PI3K/AKT signal pathway. Perhaps the IL28RA gene was regulated by lncRNA260 through the trans function.
In conclusion, lncRNA260-specific siRNA targeting IL28RA gene expression can exert the protective effect on the H/R injury cardiomyocytes by activating PI3K/Akt signaling pathway and inhibiting apoptosis. IL28RA gene promises to be a new target spot to diagnose and treat the AMI. The three pairs of siRNA with good interfering and inhibition effects on the lncRNA260 and IL28RA gene expression were found out. It would lay the foundation for exploring the lncRNA260 and IL28RA gene role in the AMI in vivo in the future.
Acknowledgements
Thank all our colleagues working in the Department of geriatrics, the First Affiliated Hospital of Nanjing Medical University.
Funding: This work was funded by the National Natural Science Foundation of China (NSFC 81100073 to Dr Yanyan Li), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University for Dr Yanyan Li (2013-2015, JX2161015034), Jiangsu Overseas Research £¶ Training Program for University Prominent Young £¶ Middle-aged Teachers and Presidents (2014) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The current research was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2009-SR-033.1).
References
- Pu J, Yuan A, Shan P, et al. Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J 2013;34:1834-5. [Crossref] [PubMed]
- Dumoutier L, Tounsi A, Michiels T, et al. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J Biol Chem 2004;279:32269-74. [Crossref] [PubMed]
- Yang L, Luo Y, Wei J, et al. Integrative genomic analyses on IL28RA, the common receptor of interferon-lambda1, -lambda2 and -lambda3. Int J Mol Med 2010;25:807-12. [PubMed]
- Yang L, Wei J, He S. Integrative genomic analyses on interferon-lambdas and their roles in cancer prediction. Int J Mol Med 2010;25:299-304. [PubMed]
- Tsai CT, Ikematsu K, Sakai S, et al. Expression of Bcl2l1, Clcf1, IL-28ra and Pias1 in the mouse heart after single and repeated administration of chlorpromazine. Leg Med (Tokyo) 2011;13:221-5. [Crossref] [PubMed]
- Sun C, Jiang H, Sun Z, et al. Identification of long non-coding RNAs biomarkers for early diagnosis of myocardial infarction from the dysregulated coding-non-coding co-expression network. Oncotarget 2016;7:73541-51. [PubMed]
- Wang Z, Zhang XJ, Ji YX, et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med 2016;22:1131-9. [Crossref] [PubMed]
- Xue Z, Hennelly S, Doyle B, et al. A G-Rich Motif in the lncRNA Braveheart Interacts with a Zinc-Finger Transcription Factor to Specify the Cardiovascular Lineage. Mol Cell 2016;64:37-50. [Crossref] [PubMed]
- Gong G, Li YY, Geng HY, et al. Protective effects of siRNA targeted IL28RA gene on hypoxia reoxygenation cardiomyocytes injury. Acta Univ Med Nanjing 2015;35:1344-8.
- Wang X, Li C, Dai Q. Down-regulation of microRNA-26b rescued hypoxia-induced apoptosis in cultured neonatal rat cardiac myocytes by regulating PTEN. Int J Clin Exp Med 2015;8:4073-9. [PubMed]
- Li YY, Zhang H, Lu XZ. Lentiviral vector PLV-PI3KCG gene transfer inhibits hypoxic cardiomyocytes apoptosis. Int J Clin Exp Med 2015;8:20208-17. [PubMed]
- Paul A, Binsalamah ZM, Khan AA, et al. A nanobiohybrid complex of recombinant baculovirus and Tat/DNA nanoparticles for delivery of Ang-1 transgene in myocardial infarction therapy. Biomaterials 2011;32:8304-18. [Crossref] [PubMed]
- Fan W, Huang J, Xiao H. Histone deacetylase 10 suppresses proliferation and invasion by inhibiting the phosphorylation of β-catenin and serves as an independent prognostic factor for human clear cell renal cell carcinoma. Int J Clin Exp Med 2015;8:3734-42. [PubMed]
- Zheng X, Lian D, Wong A, et al. Novel small interfering RNA-containing solution protecting donor organs in heart transplantation. Circulation 2009;120:1099-107. [Crossref] [PubMed]
- Zhang X, Beduhn M, Zheng X, et al. Induction of alloimmune tolerance in heart transplantation through gene silencing of TLR adaptors. Am J Transplant 2012;12:2675-88. [Crossref] [PubMed]
- Lin HB, Cadete VJ, Sra B, et al. Inhibition of MMP-2 expression with siRNA increases baseline cardiomyocyte contractility and protects against simulated ischemic reperfusion injury. Biomed Res Int 2014;2014:810371. [PubMed]
- Mordstein M, Michiels T, Staeheli P. What have we learned from the IL28 receptor knockout mouse? J Interferon Cytokine Res 2010;30:579-84. [Crossref] [PubMed]
- Chen K, Li G, Geng F, et al. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis 2014;19:946-57. [Crossref] [PubMed]
- Tabe Y, Jin L, Konopleva M, et al. Class IA PI3K inhibition inhibits cell growth and proliferation in mantle cell lymphoma. Acta Haematol 2014;131:59-69. [Crossref] [PubMed]
- Pérez-Pérez A, Gambino Y, Maymó J, et al. MAPK and PI3K activities are required for leptin stimulation of protein synthesis in human trophoblastic cells. Biochem Biophys Res Commun 2010;396:956-60. [Crossref] [PubMed]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002;2:489-501. [Crossref] [PubMed]


