Adenocarcinoma in pure ground glass nodules: histological evidence of invasion and open debate on optimal management
Lung nodules are among the most common findings associated with lung cancer. Computed tomography (CT) is the diagnostic test with the highest sensitivity for lung nodules, with wide range of size and density. Yet the specificity of CT is low in dichotomizing malignant from benign nodules, especially on the basis of a single time-point (1). Heidinger et al. analyzed a specific type of pulmonary nodule, namely the pure ground-glass nodule (pGGN) (2), which is mainly associated with histological adenocarcinoma (3). They addressed the CT characterization of resected pGGN adenocarcinoma in the three categories of stromal infiltration: adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (IAC) (3). This is one of the few reports in non-Asian population (only 10% of patients were Asian), compared to a majority of Asian studies related to the quite common surgical approach to pGGN (Table 1) (4-12). Notably, the reported results detail the CT appearance through the continuum of adenocarcinoma invasiveness and show the direct association between radiological size of pGGN and conspicuity of stromal invasion on histological specimen (e.g., number and size of invasive foci). The larger the nodule the larger the invasion of adenocarcinoma, yet small size of pGGN does not warrant absence of infiltration: foci of invasion were seen in almost 10% of adenocarcinomas within pGGN <10 mm, including a 5% of IAC. Nevertheless, it should be underscored that this population was gauged to histologically confirmed adenocarcinoma, hence the proportion of invasion is expected to drop when including resected pGGN with histological evidence of pre-malignant lesion (e.g., atypical adenomatous hyperplasia) or benign finding (e.g., focal fibrosis).
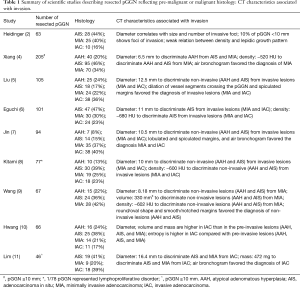
Full table
In this series, the association between pGGN size and stromal infiltration was substantially similar for manual caliper and manual volume. This observation offers a practical approach, which is particularly convenient for the clinical practice: manual caliper is the simplest and most available method for nodule quantification (13), meanwhile it seems to be as good as volumetry for risk stratification, the latter is a more complex method and has limited availability in the clinical workflow. As the authors report, the manual caliper finds its application in the single time-point assessment of the pGGN. Conversely, pGGN density at a single time-point was not associated with conspicuity of stromal infiltration. This observation confirms the results from previous studies (10,14). Alternative quantitative metrics of pGGN density (e.g., entropy, homogeneity) were found to be predictive of malignancy, yet their analytic nature, the absence of consolidated threshold, and the issues with standardization still prevent an intuitive implementation in clinical practice (10). In contrast to this set of relatively simple quantitative metrics, there is evidence that visual classification of minor density difference is associated with nodule malignancy (15). For instance, subsolid nodules with subtle solid component on the sole lung window might be more likely to grow to overt part-solid nodules with increased risk of malignancy (15). Beyond size and density characterization, visual features of pGGN are predictors of malignancy, for instance, sharpness of the pGGN margin, air bronchogram, and abnormality of the adjacent vessel (7,14). Guidelines for nodule management include qualitative descriptors features that increase the suspicion of malignancy (e.g., effect on surrounding tissue, internal nodule structure, border characteristics, etc.) (16). However, there are known limitations from the inter- and intra-observer variability in such qualitative features of pulmonary nodules (17). The attempt to reduce subjective variability in the definition of such qualitative features is being endorsed by software developers because subtle morphological differences in pGGN can play a significant role in prognostication, and it is particularly relevant when assessing the nodule on a single time-point. Translation of morphologic descriptors of nodule from visual to computerized analysis was reported to be extremely accurate for risk stratification of both pGGN (18) and other types of nodule (19).
The importance of risk stratification of pGGN follows the progressive increase in detection of nodules by CT (20). Management of pGGN is particularly debated between resection or active surveillance (21,22). Single time-point assessment and short-term follow up are discouraged for pGGN (23) because a sizeable proportion of such nodules maintain growth potential even after several years of stability (24). The surgical yield of pGGN is relatively low and resection of <10 mm pGGN might have overall clinical detrimental effect, even in case of ascertained adenocarcinoma (25). For instance, surgical resection of a centrally located pGGN might technically require lobectomy, which is disproportionate compared to the clinical risk of clinical relevance of pGGN. Overall, a pGGN on CT has relatively slow progression rate even when it represents adenocarcinoma (26), therefore it may be included in the “reservoir” of clinically silent malignancy, like it is known for indolent lesions of epithelial origin (IDLE) of thyroid or prostate (27,28). Therefore, the subtle clinical balance of pGGN management should be carefully pursued by active surveillance (Figure 1). The reports from lung cancer screening trials showed that conservative management of pGGN provides optimal balance between risk and benefits. In asymptomatic subjects at high risk of lung cancer, pGGN did not progress to advanced stage lung cancer in several years after first documentation on CT (29-32). Therefore, long-term active surveillance by low-dose CT (LDCT) is encouraged until the evidence of nodule progression. Heidinger et al. report almost 50% of AIS among the resected pGGN, hence the questions are: how many of these patients might have been managed with active surveillance in spite of resection? Shall manual caliper be trusted for longitudinal assessment of pGGN in clinical practice? So far, it has been shown that density and volume can provide comprehensive characterization in the longitudinal assessment of subsolid nodules, including pGGN (10,33). The mass doubling time (MDT) was proposed as the most appropriate radiological predictor of malignancy in the assessment of pGGN by multiple LDCT time-points (33,34). MDT is calculated by a combination of volume, density and time, which allows quantitative comprehensive view on the nodule evolution (33). For instance, pGGN may increase in mass despite a reduction in volume, in this case MDT is a robust indicator of overall nodule growth. It was reported that MDT is independent from the nodule features at first detection (35). Furthermore, the appearance of a solid component is quoted as good trigger to surgical action, without survival decrease (22). Lee et al. recently reported the strategy of “follow-up and surgical action after interval growth”, even for part-solid nodules which are known to have the strongest association with malignancy compared to any other kind of pulmonary nodule (22). In this regard, it is fostered that future studies will test temporal characterization of pGGN by simple CT metrics in the histological continuum of pulmonary adenocarcinoma. In particular, the multi time-point evolution of manual caliper and density should be tested against volumetric parameters for characterization of AIS, MIA, and IAC. From the clinical perspective (e.g., out of the highly specialized workflow of lung cancer screening), it is interesting to understand how far the manual caliper should be trusted for pGGN monitoring and which cases might take advantage of more complex radiological characterization.
Finally, there is evidence that subjects with lung adenocarcinoma are more likely to develop a second lung cancer (36). Also, non-pulmonary oncologic comorbidities or non-oncologic comorbidities (e.g., cardiovascular disease, chronic obstructive pulmonary disease, etc.) may hamper the clinical relevance of pGGN (25). Given the indolent nature of stable pGGN, its conservative management should be pursued until the evidence of growth progression with the aim of reducing the pulmonary function damage (22).
In conclusion, the awareness that pGGN of any size may harbor foci of stromal invasion should not panic the thoracic oncology multi-disciplinary team, yet it should drive the management of pGGN through active surveillance by LDCT. The correlation between manual caliper and extent of stromal infiltration does not warrant absence of invasion in smaller nodules, yet signs of growth during surveillance are expected to point out pGGN with more aggressive behavior. Simple parameters for clinical practice are fostered for the longitudinal characterization and management of such lesions with relatively low rate of progression.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Suzuki K. Whack-a-mole strategy for multifocal ground glass opacities of the lung. J Thorac Dis 2017;9:S201-7. [Crossref] [PubMed]
- Heidinger BH, Anderson KR, Nemec U, et al. Lung adenocarcinoma manifesting as pure ground-glass nodules: Correlating CT size, volume, density, and roundness with histopathologic invasion and size. J Thorac Oncol 2017;12:1288-98. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Xiang W, Xing Y, Jiang S, et al. Morphological factors differentiating between early lung adenocarcinomas appearing as pure ground-glass nodules measuring</=10 mm on thin-section computed tomography. Cancer Imaging 2014;14:33. [Crossref] [PubMed]
- Liu LH, Liu M, Wei R, et al. CT findings of persistent pure ground glass opacity: can we predict the invasiveness? Asian Pac J Cancer Prev 2015;16:1925-8. [Crossref] [PubMed]
- Eguchi T, Yoshizawa A, Kawakami S, et al. Tumor size and computed tomography attenuation of pulmonary pure ground-glass nodules are useful for predicting pathological invasiveness. PLoS One 2014;9:e97867. [Crossref] [PubMed]
- Jin X, Zhao SH, Gao J, et al. CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground-glass opacity. Eur Radiol 2015;25:2532-40. [Crossref] [PubMed]
- Kitami A, Sano F, Hayashi S, et al. Correlation between histological invasiveness and the computed tomography value in pure ground-glass nodules. Surg Today 2016;46:593-8. [Crossref] [PubMed]
- Wang X, Wang L, Zhang W, et al. Can we differentiate minimally invasive adenocarcinoma and non-invasive neoplasms based on high-resolution computed tomography features of pure ground glass nodules? PLoS One 2017;12:e0180502. [Crossref] [PubMed]
- Hwang IP, Park CM, Park SJ, et al. Persistent Pure Ground-Glass Nodules Larger Than 5 mm: Differentiation of Invasive Pulmonary Adenocarcinomas From Preinvasive Lesions or Minimally Invasive Adenocarcinomas Using Texture Analysis. Invest Radiol 2015;50:798-804. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules >/= 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Li M, Gao F, Jagadeesan J, et al. Incremental value of contrast enhanced computed tomography on diagnostic accuracy in evaluation of small pulmonary ground glass nodules. J Thorac Dis 2015;7:1606-15. [PubMed]
- Bankier AA, MacMahon H, Goo JM, et al. Recommendations for Measuring Pulmonary Nodules at CT: A Statement from the Fleischner Society. Radiology 2017.162894. [Crossref] [PubMed]
- Wu F, Tian SP, Jin X, et al. CT and histopathologic characteristics of lung adenocarcinoma with pure ground-glass nodules 10 mm or less in diameter. Eur Radiol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- Chung K, Jacobs C, Scholten ET, et al. Lung-RADS Category 4X: Does It Improve Prediction of Malignancy in Subsolid Nodules? Radiology 2017;284:264-71. [Crossref] [PubMed]
- van Riel SJ, Sanchez CI, Bankier AA, et al. Observer Variability for Classification of Pulmonary Nodules on Low-Dose CT Images and Its Effect on Nodule Management. Radiology 2015;277:863-71. [Crossref] [PubMed]
- Nemec U, Heidinger BH, Anderson KR, et al. Software-based risk stratification of pulmonary adenocarcinomas manifesting as pure ground glass nodules on computed tomography. Eur Radiol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Ciompi F, Chung K, van Riel SJ, et al. Towards automatic pulmonary nodule management in lung cancer screening with deep learning. Sci Rep 2017;7:46479. [Crossref] [PubMed]
- Gould MK, Tang T, Liu IL, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med 2015;192:1208-14. [Crossref] [PubMed]
- Patz EF Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269-74. [Crossref] [PubMed]
- Lee JH, Park CM, Kim H, et al. Persistent part-solid nodules with solid part of 5 mm or smaller: Can the 'follow-up and surgical resection after interval growth' policy have a negative effect on patient prognosis? Eur Radiol 2017;27:195-202. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Cho J, Kim ES, Kim SJ, et al. Long-Term Follow-up of Small Pulmonary Ground-Glass Nodules Stable for 3 Years: Implications of the Proper Follow-up Period and Risk Factors for Subsequent Growth. J Thorac Oncol 2016;11:1453-9. [Crossref] [PubMed]
- Gulati CM, Schreiner AM, Libby DM, et al. Outcomes of unresected ground-glass nodules with cytology suspicious for adenocarcinoma. J Thorac Oncol 2014;9:685-91. [Crossref] [PubMed]
- Kakinuma R, Muramatsu Y, Kusumoto M, et al. Solitary Pure Ground-Glass Nodules 5 mm or Smaller: Frequency of Growth. Radiology 2015;276:873-82. [Crossref] [PubMed]
- Esserman LJ, Thompson IM Jr, Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA 2013;310:797-8. [Crossref] [PubMed]
- Zha J, Xie D, Xie H, et al. Recognition of "aggressive" behavior in "indolent" ground glass opacity and mixed density lesions. J Thorac Dis 2016;8:1460-8. [Crossref] [PubMed]
- Infante M, Lutman RF, Imparato S, et al. Differential diagnosis and management of focal ground-glass opacities. Eur Respir J 2009;33:821-7. [Crossref] [PubMed]
- Silva M, Sverzellati N, Manna C, et al. Long-term surveillance of ground-glass nodules: evidence from the MILD trial. J Thorac Oncol 2012;7:1541-6. [Crossref] [PubMed]
- Scholten ET, de Jong PA, de Hoop B, et al. Towards a close computed tomography monitoring approach for screen detected subsolid pulmonary nodules? Eur Respir J 2015;45:765-73. [Crossref] [PubMed]
- Yip R, Yankelevitz DF, Hu M, et al. Lung Cancer Deaths in the National Lung Screening Trial Attributed to Nonsolid Nodules. Radiology 2016;281:589-96. [Crossref] [PubMed]
- de Hoop B, Gietema H, van de Vorst S, et al. Pulmonary ground-glass nodules: increase in mass as an early indicator of growth. Radiology 2010;255:199-206. [Crossref] [PubMed]
- Song YS, Park CM, Park SJ, et al. Volume and mass doubling times of persistent pulmonary subsolid nodules detected in patients without known malignancy. Radiology 2014;273:276-84. [Crossref] [PubMed]
- Borghesi A, Farina D, Michelini S, et al. Pulmonary adenocarcinomas presenting as ground-glass opacities on multidetector CT: three-dimensional computer-assisted analysis of growth pattern and doubling time. Diagn Interv Radiol 2016;22:525-33. [Crossref] [PubMed]
- Han S, Rivera GA, Cheng I, et al. PS01.77: Risk-Stratification for Second Primary Lung Cancer: Topic: Medical Oncology. J Thorac Oncol 2016;11:S319-20. [Crossref] [PubMed]


