Recent advances in radiotherapy for thoracic tumours
Non-small cell lung cancer—staging
One cannot consider radiotherapy advances without first evaluating the impact of imaging. We should note the staging changed with the introduction of TNM version 7 following a large multinational, multi-disciplinary, and international collaboration (1). Clearly, avoiding treating patients with metastatic disease is beneficial and improves the cost effectiveness of treatment (2). However, we have reached another level of PET use with the rational integration of functional imaging data into radiotherapy planning. It makes little sense to try to treat possible subclinical disease when you are unable to control the primary tumour (David Ball—personal communication). Indeed trying to treat larger volumes may actually impair outcomes by compromising dose.
Waiting time continues to receive attention. Earlier studies have shown that delay is associated with larger tumour volumes at treatment (3). Most recently the effect of delay on the extent of the disease on PET volumes has been examined (4). When patients were subjected to a staging PET and an RT planning PET it was evident that the mean tumour volume had almost doubled on PET. 6/82 patients were then unsuitable for radical treatment (4).
EBUS/TBNA has had an effect here as well. While FDG PET is exquisitely sensitive it is not 100% specific; the presence of tuberculosis significantly complicates the analysis for example. Recent studies of regional nodes suggest TBNA in instead of PET may improve staging accuracy (5).
PET-MR is the next major clinically available advance in imaging technology. Concurrent acquisition of PET data and MR imaging has presented significant technical challenges as the whole method of acquiring a PET image has had to be re-engineered (6,7). The presence of a magnetic field however, limits the range of positrons thereby increasing in intrinsic resolution of PET-MR when compared to conventional PET. This technology is in the early stages of clinical adoption.
In-situ disease (CIS)/minimally invasive disease
Bronchial brachytherapy has attracted some interest with advances in bronchoscopic technique and technical improvements such as bronchoscopic ultrasound (EBUS) allowing a unique view of the tumour.
Brachytherapy is being employed using bronchoscopically placed catheters and an iridium HDR source. Bronchoscopic advances such as ultrasound have assisted in the definition of tumour volume and defining the edges of tumour to be treated.
Managing movement of the tumour and organs at risk
Various techniques exist to account for tumour movement, both during planning and treatment. Fiducial markers are one such solution that is useful throughout. Implanted fiducial markers present an opportunity to better define tumour outline at the planning stage and provide a ‘geometric fix’ on a tumour during therapy, when coupled with real-time imaging modalities. Advances here are happening concurrently with technical developments in bronchoscopy and ultrasound.
A robotically controlled linear accelerator (Cyberknife™, Accuray) solves the online movement problem by moving the radiation source in sync with the target. Diagnostic X-rays are used to close the feedback loop with the linear accelerator, constantly updating it with the position of the target. Another such online target tracking system is Calypso (Calypso Medical), which uses radio frequency transponders as fiducial markers.
Offline gating presents yet another solution to the problem allowing for more precise target definition. Here the CT puts the images into “bins” according to the phase of the breathing cycle. Total scan time is increased but useful position data can be acquired; thus a “4D CT” is generated. Online gating systems are also available for target motion compensation during treatment by tracking the motion of the patients external contour, such as Varian RPM. Systems such as this however, track a surrogate of the target motion, not the target motion itself.
Another example of motion compensation is to use a PET fused to the planning CT. As the PET is acquired over about 20 mins it smears out the tumour volume effectively defining a region in which the tumour is most likely to reside.
4D CT (and 4D-PET) have been looked at as a way of defining tumour motion which may be more accurate than our usual geometric expansions. Finally coaching of patients using some form of bio-feedback is finding increasing clinical application with the same aim.
Volume definition
With better technology telling us where to treat; so have come RT advances allowing us to treat small and moving targets. The concept of “volumetric conformity” still has significant difficulties with implementation. At present automated methods for tumour delineation have not proven robust enough for clinical use.
PET imaging with 18FDG has revolutionised both the staging and treatment volume definition but problems remain. Standardised uptake values (SUV) are not standardised between machines and edges of the tumour remain difficult to define (8-10). Modelling has been undertaken looking at the changes in dose to critical normal tissues. This shows PET decreases the dose delivered to normal tissue; while improving the tumour control probability (11).
Cone beam CT (or tomotherapy megavoltage CT) present the possibility of adjusting tumour volume definitions during treatment as the tumour shrinks (12). Such approaches appear to decrease the volume of normal tissue irradiated (13).
Finally automated target volume definition, again with FDG, has been attempted. As yet there is little agreement with “manually derived” contours (14). The technology is still immature but may one day allow daily changes in treatment volume without prohibitive cost.
Treatment response
Advances in imaging have allowed changes to the irradiated volume to occur even on a daily basis (15).
A pilot study of interval PET has shown that a PET two weeks into treatment can be useful in terms of defining response to radiotherapy (16). Further modifications will no doubt examine dose painting to boost areas of greater tumour activity. While we doubt any oncologist would stop treatment early in the course of treatment it may be a prompt to increase dose or intensify treatment.
Intensity modulated radiotherapy (IMRT) techniques such as helical tomotherapy have allowed us to “bend” the dose cloud around critical structures. The role of tomotherapy would seem to be particularly in central and posteriorly placed tumours where the radiation oncologist is trying to avoid critical structures. It may have a role in treating multiple primary tumours or RT plans in which very large volumes of normal tissue are being irradiated. The role of dose painting and dose escalation continues to receive research attention.
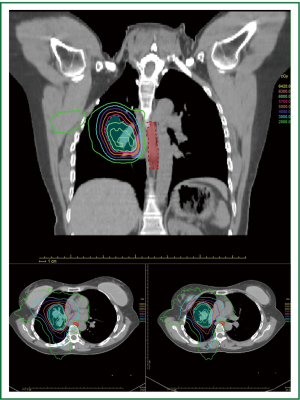
Planning studies suggest the dose uniformity and homogeneity may be better with tomotherapy™ but the area irradiated to low dose is probably increased (13). Tomotherapy™ has been evaluated in studies looking at integral dose and the risk of induced malignancies (17). This is thought to be no greater than other highly conformal techniques (Figure 1).
Hypofractionation using stereotactic body radiotherapy
Probably the largest clinical impact has come from hypo-fractionating treatment. The biological effect of RT is significantly increased by giving a small number of large fractions (so called hypofractionation). Often this is less than five fractions. The work of Timmerman and others have highlighted the importance of a biologically equivalent dose (BED) of at least 150 Gy (18,19). Given the significant changes in patterns of care which are happening, it is surprising there is not a wealth of randomised clinical trials (20). The practical implications of implementing such a change to treatment paradigms should not be forgotten (21).
Central tumours have caused some concern that toxicity would be increased but a recent systematic review has not borne this out (22). As long as an appropriate fractionation schedule is employed the toxicity appears manageable and efficacy maintained. Imaging is even more important for hypofractionation, and especially with regard to motion compensation.
Protons have been employed in hypofractionated lung treatment although cost remains prohibitive in many countries (23).
Investigators have looked at minimally invasive disease treated with stereotactic radiotherapy (24). Fitting with the concept of tumours formerly referred to as bronchiolo-alveloar carcinoma (BAC) as a field change there were concerns that there would be potential difficulties with defining the edges of the tumour. Interestingly however, the there was no significant difference noted in three year regional failure.
Tumour volume
Tumour volume has been investigated by the Trans-Tasman Radiation Oncology Group (TROG). Previously tumour size was not shown to correlate with clinical stage (25) and more recent work has shown the relationship to prognosis is complex. Indeed the new staging system makes little mention of tumour size (26). The prognostic significance of tumour size changes over time—in the first 18 months the larger the tumour the higher risk of dying. Beyond 18 months the association is weak and the authors suggest size alone should not be a reason to deny a patient potentially curative treatment (26).
Locally advanced disease
The advances here are likely to be from combinations of chemotherapy or combinations with molecular agents. We need better tools to quantify the effect of low doses of radiation on normal lung tissue. The high dose region is usually able to be smaller and more conformal but problems still remain.
In locally advanced disease the challenge lies in minimising volumes of normal lung irradiated while covering all the tumour and doing so at a dose high enough to sterilise the area. New approaches to advanced disease include the addition of biologic agents such as cetuximab (27,28) as they have in other sites. This is based on preclinical models suggesting a radiosensitisation effect (27). This treatment has modest additional benefit.
Molecular markers
The molecular revolution has not escaped this corner of medicine. TGF-beta isoforms are thought to be related to the risk of radiation pneumonitis. The relationship between TGF-beta and the development of pneumonitis appears complex and ongoing efforts aim to refine predictors of radiation pneumonitis (29).
Radiation pneumonitis has also been associated with genetic variation in the form of single nucleotide polymorphisms (SNPs) for certain genotypes of heat shock proteins associated with a greatly increased risk of radiation pneumonitis in non small cell lung cancers treated with chemoradiation (30).
Radiogenomic studies (31) ave pointed to promising areas of research aimed at predicting response to combined modality therapy. Early in vitro early evidence has emerged of anaplastic lymphoma kinase (ALK) inhibitors enhancing radiotherapy response (32). In vivo models show some promise with concurrent use of hedgehog pathway inhibitors (33).
Small cell lung cancer
PET imaging with 18FDG for small cell lung cancer has been examined in a systematic review (34). Cost appeared comparable—at least in the Australian context. Radiotherapy changes, such as changed field borders, resulted in changes in about 28% of patients. About 6% of small cell lung cancer patients would be offered RT after a PET who would not have been offered RT prior to PET. A further 9% of patients with occult metastatic disease would be spared radical treatment.
We have phase III evidence supporting the benefit of hyperfractionated accelerated radiotherapy but the uptake of this seems slow in practice—perhaps reflecting the difficulty in getting patients though such a regimen. Perhaps here is a further application of IMRT treatment.
Mesothelioma—pleural radiotherapy
IMRT techniques have been widely used in management of resected and unresected malignant pleural mesothelioma (35-37). There is as yet no consensus on its role as a routine standard of care (38).
There are some reports that conventional lung normal tissue constraints using V20 and Mean Lung Dose MLD are not appropriate after extrapleural pneumonectomy and that more conservative constraints using V5 are needed (39).
An Italian study has reported in abstract form reporting the use of accelerated hypofractionation over 5 fractions with helical Tomotherapy for unresected mesothelioma with acceptable toxicity (40). An Australian study reported 71% infield local control included PET based Total Glycolytic Volume as well as survival outcome data using IMRT (41).
Conclusions
It is fortunate that emerging health technologies are set to change the way we implement radiation oncology practice to achieve the best outcomes for our patients with lung cancer. Nonetheless, despite the hope and promise of new technologies we should not forget the effect that our treatments have on our patient’s quality of life. As these new tools allow us to do more—we hope that we will be better able to choose patients for treatment, adapt that treatment to them and that with more conformal treatment related toxicity will reduce (42).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet 2011;378:1727-40. [PubMed]
- Kalff V, Hicks RJ, MacManus MP, et al. Clinical impact of (18)F fluorodeoxyglucose positron emission tomography in patients with non-small-cell lung cancer: a prospective study. J Clin Oncol 2001;19:111-8. [PubMed]
- O'Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12:141-4. [PubMed]
- Everitt S, Plumridge N, Herschtal A, et al. The impact of time between staging PET/CT and definitive chemo-radiation on target volumes and survival in patients with non-small cell lung cancer. Radiother Oncol 2013;106:288-91. [PubMed]
- Kuo CH, Chen HC, Chung FT, et al. Diagnostic value of EBUS-TBNA for lung cancer with non-enlarged lymph nodes: a study in a tuberculosis-endemic country. PLoS One 2011;6:e16877. [PubMed]
- Chandarana H, Heacock L, Rakheja R, et al. Pulmonary Nodules in Patients with Primary Malignancy: Comparison of Hybrid PET/MR and PET/CT Imaging. Radiology 2013. [Epub ahead of print]. [PubMed]
- Schwenzer NF, Schraml C, Müller M, et al. Pulmonary lesion assessment: comparison of whole-body hybrid MR/PET and PET/CT imaging--pilot study. Radiology 2012;264:551-8. [PubMed]
- Suzawa N, Ito M, Qiao S, Uchida K, et al. Assessment of factors influencing FDG uptake in non-small cell lung cancer on PET/CT by investigating histological differences in expression of glucose transporters 1 and 3 and tumour size. Lung Cancer 2011;72:191-8. [PubMed]
- MacManus M, Nestle U, Rosenzweig KE, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006-2007. Radiother Oncol 2009;91:85-94. [PubMed]
- Yaremko B, Riauka T, Robinson D, et al. Thresholding in PET images of static and moving targets. Phys Med Biol 2005;50:5969-82. [PubMed]
- van Der Wel A, Nijsten S, Hochstenbag M, et al. Increased therapeutic ratio by 18FDG-PET CT planning in patients with clinical CT stage N2-N3M0 non-small-cell lung cancer: a modeling study. Int J Radiat Oncol Biol Phys 2005;61:649-55. [PubMed]
- Knap MM, Hoffmann L, Nordsmark M, et al. Daily cone-beam computed tomography used to determine tumour shrinkage and localisation in lung cancer patients. Acta Oncol 2010;49:1077-84. [PubMed]
- Meng LL, Feng LC, Wang YL, et al. Dosimetric comparison between helical tomotherapy and intensity-modulated radiation therapy plans for non-small cell lung cancer. Chin Med J (Engl) 2011;124:1667-71. [PubMed]
- Niyazi M, Landrock S, Elsner A, et al. Automated biological target volume delineation for radiotherapy treatment planning using FDG-PET/CT. Radiat Oncol 2013. [Epub ahead of print]. [PubMed]
- Dobbs HJ. Defining the radiation target on a daily basis. Cancer Imaging 2006;6:30-2. [PubMed]
- Kong FM, Frey KA, Quint LE, et al. A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol 2007;25:3116-23. [PubMed]
- Kim DW, Chung WK, Shin D, et al. Risk of second cancer from scattered radiation of intensity-modulated radiotherapies with lung cancer. Radiat Oncol 2013;8:47. [PubMed]
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003;124:1946-55. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Senan S. Surgery versus stereotactic radiotherapy for patients with early-stage non-small cell lung cancer: More data from observational studies and growing clinical equipoise. Cancer 2013;119:2668-70. [PubMed]
- Dahele M, Pearson S, Purdie T, et al. Practical considerations arising from the implementation of lung stereotactic body radiation therapy (SBRT) at a comprehensive cancer center. J Thorac Oncol 2008;3:1332-41. [PubMed]
- Senthi S, Haasbeek CJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013;106:276-82. [PubMed]
- Hata M, Tokuuye K, Kagei K, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys 2007;68:786-93. [PubMed]
- Badiyan SN, Bierhals AJ, Olsen JR, et al. Stereotactic body radiation therapy for the treatment of early-stage minimally invasive adenocarcinoma or adenocarcnioma in situ (formerly bronchioloalveolar carcinoma): a patterns of failure analysis. Radiat Oncol 2013;8:4. [PubMed]
- Ball DL, Fisher R, Burmeister B, et al. Stage is not a reliable indicator of tumor volume in non-small cell lung cancer: a preliminary analysis of the Trans-Tasman Radiation Oncology Group 99-05 database. J Thorac Oncol 2006;1:667-72. [PubMed]
- Ball DL, Fisher RJ, Burmeister BH, et al. The complex relationship between lung tumor volume and survival in patients with non-small cell lung cancer treated by definitive radiotherapy: a prospective, observational prognostic factor study of the Trans-Tasman Radiation Oncology Group (TROG 99.05). Radiother Oncol 2013;106:305-11. [PubMed]
- Blumenschein GR Jr, Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol 2011;29:2312-8. [PubMed]
- Jensen AD, Münter MW, Bischoff H, et al. Treatment of non-small cell lung cancer with intensity-modulated radiation therapy in combination with cetuximab: the NEAR protocol (NCT00115518). BMC Cancer 2006;6:122. [PubMed]
- Vujaskovic Z, Groen HJ. TGF-beta, radiation-induced pulmonary injury and lung cancer. Int J Radiat Biol 2000;76:511-6. [PubMed]
- Pang Q, Wei Q, Xu T, et al. Functional promoter variant rs2868371 of HSPB1 is associated with risk of radiation pneumonitis after chemoradiation for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;85:1332-9. [PubMed]
- Das AK, Bell MH, Nirodi CS, et al. Radiogenomics predicting tumor responses to radiotherapy in lung cancer. Semin Radiat Oncol 2010;20:149-55. [PubMed]
- Dai Y, Melzig C, Hanne J, et al. Combined ALK-inhibition and Radiation Therapy in Lung Cancer. International Journal of Radiation Oncology*Biology*Physics. Elsevier Inc, 2012;84:S706.
- Zeng J, Aziz K, Chettiar ST, et al. Hedgehog pathway inhibition radiosensitizes non-small cell lung cancers. Int J Radiat Oncol Biol Phys 2013;86:143-9. [PubMed]
- Ruben JD, Ball DL. The efficacy of PET staging for small-cell lung cancer: a systematic review and cost analysis in the Australian setting. J Thorac Oncol 2012;7:1015-20. [PubMed]
- Rosenzweig KE, Zauderer MG, Laser B, et al. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2012;83:1278-83. [PubMed]
- Patel PR, Yoo S, Broadwater G, et al. Effect of increasing experience on dosimetric and clinical outcomes in the management of malignant pleural mesothelioma with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:362-8. [PubMed]
- Ebara T, Kawamura H, Kaminuma T, et al. Hemithoracic intensity-modulated radiotherapy using helical tomotherapy for patients after extrapleural pneumonectomy for malignant pleural mesothelioma. J Radiat Res 2012;53:288-94. [PubMed]
- Chapman E, Berenstein EG, Diéguez M, et al. Radiotherapy for malignant pleural mesothelioma. Cochrane Database Syst Rev 2006;CD003880. [PubMed]
- Allen AM, Czerminska M, Jänne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys 2006;65:640-5. [PubMed]
- Parisi E, Sarnelli A, Giannini M, et al. Accelerated Hypofractionated Radiation Therapy Using Helical Tomotherapy for the Treatment of Medically Inoperable Pleural Mesothelioma: IRST Preliminary Data. International Journal of Radiation Oncology*Biology*Physics. Elsevier Inc, 2012;84:S578-S9.
- Feigen M, Lee ST, Lawford C, et al. Establishing locoregional control of malignant pleural mesothelioma using high-dose radiotherapy and (18) F-FDG PET/CT scan correlation. J Med Imaging Radiat Oncol 2011;55:320-32. [PubMed]
- McCloskey P, Balduyck B, Van Schil PE, et al. Radical treatment of non-small cell lung cancer during the last 5 years. Eur J Cancer 2013;49:1555-64. [PubMed]