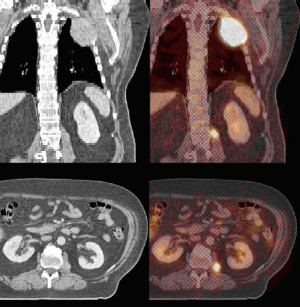
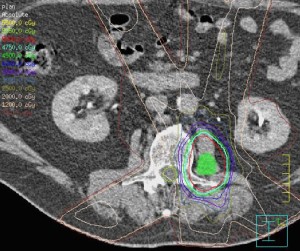
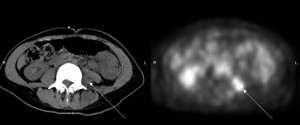
This case series presents three patients with NSCLC metastatic
to the psoas muscle. In two patients, the histology was squamous
carcinoma, and one case was an adenocarcinoma. Each patient
was treated with RT to the involved area, in addition to systemic
chemotherapy. Two patients had isolated psoas metastases
and were treated with curative intent for oligometastatic
disease; one patient has a single psoas metastasis in addition
to bony metastases and was treated palliatively. Ultimately,
all three patients developed widespread metastases and two
have succumbed to their disease, a finding reflecting the poor
prognosis associated with oligometastatic NSCLC and a locally
advanced primary (
3). However, with follow-up ranging from 8
to 15 months after psoas irradiation, all three patients tolerated
treatment well and remained entirely asymptomatic with respect
to the psoas lesions.
Case reports of the metastatic spread of cancer to the psoas
muscle, though rare, date back over a century, including one
case from a pancreas primary published by Flexner in 1894
(
4). There are only a few cases in the literature reporting direct
hematogenous metastasis of lung cancer to the psoas muscle.
Ampil et al. describe seven cases of metastatic tumors involving
the psoas muscle, including one from a lung primary (
5). In two
patients, RT to the psoas metastasis provided effective palliation
of pain. Nash et al. reported on a single case of a biopsy proven
psoas metastasis from an adenocarcinoma of the lung (
6).
Damron et al. presented 30 cases of cancer metastatic to soft tissue; this series included one patient with small cell lung cancer
metastatic to the psoas muscle (
7). Sudo et al. described a case
of adenocarcinoma of the lung metastatic to the psoas muscle
in a patient presenting with pain on hip extension (
8). Kenny et
al. described 25 cases of psoas muscle invasion by malignancy
including one adenocarcinoma of a lung primary (
9). Of these
cases only two represented hematogenous metastasis. Lenchik
et al. reported on 44 cases of CT identified psoas abnormalities
of which 15 were malignancies, two from a primary lung cancer
(
10). These published series are summarized in
Table 1.
Published reports on the use of CT or MRI also suggest
that anatomic imaging cannot accurately identif y psoas
muscle pathology (
10,
11,
12). Cases of metastatic disease
masquerading as benign processes such as psoas abscesses have
been reported (
13,
14). While it is possible that these findings
could be explained by benign processes or synchronous primary
malignancies, this possibility is remote. The three psoas lesions
described in this series are unlikely to represent any other
disease process except hematogenous metastatis of lung cancer.
This contention is supported by several findings: the absence
of preceding trauma, the absence of the stigmata of infection,
the appearance of psoas masses on CT scans (in two patients),
correlated hypermetabolic activity on FDG-PET (in three
patients), the resolution of these radiographic findings after RT,
and the clinical setting of advanced NSCLC. Given the expense
and possible morbidity of surgical biopsy balanced against
the high likelihood that these lesions represented metastatic
NSCLC, the risks of pathologic confirmation were not deemed
to be justified.
Based on the relative paucity of published reports, it appears
that very few psoas metastases become radiographically
apparent or clinically symptomatic. By contrast, autopsy studies
suggest that metastatic infiltration of the psoas muscles is far
more common. In one Spanish study, 194 patients with known
malignancy underwent postmortem examination (
15). At
autopsy, 50 were found to have involvement of skeletal muscle
with cancer, 16 by direct extension, 34 by metastastic spread.
The muscle groups most often involved were the diaphragm
(23 patients) and the iliopsoas (10 patients), although this
distribution may, in part, represent a sampling bias since
these muscle groups were sectioned more often than others.
Adenocarcinoma appeared to metastasize to skeletal muscle more frequently than other cancer histologies. Another study
of patients with a diagnosis of malignancy found metastases in
skeletal muscle in six out of 38 autopsies (
16). In four of these
cases, cancer was found in the iliopsoas muscles.
It is not known how to reconcile the discrepancy between
the rarity of clinically apparent psoas metastases and the high
incidence of detection on autopsy, however, the “seed and soil”
hypothesis may provide some insight. Paget postulated that the
formation of metastases depends on the interaction of specially
adapted tumor cells (seeds) and a permissive organ milieu
(soil) (
17). In more recent formulations of this theory, tumor
cells must accumulate specific genetic mutations endowing
them with the metastatic phenotype. Once tumor cells acquire
the ability to intravasate into the vasculature, they disseminate
hematogenously and enter the parenchyma of target organs.
Tumor cells then face diverse cellular microenvironments that
range from permissive to hostile. Only those cells that are well
suited to overcoming obstacles to growth, and find themselves
in a suitable milieu, will develop into metastases. Tumor cells in
less permissive environments may die or grow in a more indolent
fashion (
18). It is this set of complex interactions between tumor
cells and distant organs that underlies disease specific patterns of
distant failure.
The “seed and soil” hypothesis has two impor tant
implications for the observations in the present study. First, it
explains the finding, universal to all malignant processes, that
distant metastases tend to be unevenly distributed throughout
the body (
19). In the case of lung cancer, clinically evident psoas
metastases are extremely rare because the cellular environment
of skeletal muscle is less hospitable than other organs. Thus,
the interactions between tumor cells and target tissues lead
to the preferential development of metastatic disease in more
permissive tissue such as the brain, bone and adrenal glands.
Second, the “seed and soil” hypothesis may account for the
seemingly contradictory observations that metastases to the
psoas muscle are rarely identified clinically but are commonly
found on autopsy. Due to the resistance of skeletal muscle to
tumor implantation, psoas metastases may progress more slowly
than tumor deposits elsewhere in the body or present later in
the course of disease. The advent of PET imaging may reveal
psoas metastases that would have otherwise gone undetected. If
true, then the frequency of the detection of psoas metastases in
NSCLC may increase in the future.
In the cases presented above, RT was used to treat all
psoas metastases. This decision hinged on the poor prognosis
associated with skeletal metastasis in lung cancer as well as the
morbidity of surgical resection of psoas lesions.
Table 1. Published series of clinically detected psoas metastases from lung cancer
| Study |
# Cases of psoas lesions |
# Cases of malignancy |
# Cases of lung malignancy |
| Ampil et al (5) |
7 |
7 |
1 |
| Nash et al (6) |
1 |
1 |
1 |
| Damron et al (7) |
1 |
1 |
1 |
| Sudo et al (8) |
1 |
1 |
1 |
| Kenny et al (9) |
25 |
2 |
1 |
|
|
|
|