Pneumonectomy in pulmonary metastasis
Introduction
Pneumonectomy is considered to be a high risk surgery, often accompanied by complications; therefore, a thorough review is required prior to deciding to perform pneumonectomy (1-5). In 1999, the International Registry of Lung Metastases (IRLM) reported 171 patients (3.2%) received pneumonectomy, among 5,206 patients who underwent metastasectomy, with hospital mortality rate of 5.2% and 5-year survival rate of 20% (5). The IRLM report was the only available large scale multicenter study conducted on metastatic lung tumors. We retrospectively analyzed data obtained from multiple institutions on patients who underwent the surgery for metastatic lung tumors in recent years and evaluated the pros and cons of pneumonectomy.
Methods
The Metastatic Lung Tumor Study Group of Japan has conducted a registry of metastatic lung tumor cases that are treated with surgical resection (6). To date, 25 institutions have joined the group, and 4,742 patients were registered between 1984 and 2013. This study was approved by the Institutional Ethics Review Board (Teikyo University Review Board 10–108) and other institutions, and waived the need for informed consent from patients, as long as patient data remained anonymous. The following parameters were collected: gender, age, histology of primary tumor, staging of primary tumor, treatment for the primary tumor, date of primary surgery, type of surgery, curability, disease-free interval (DFI), side of pulmonary metastasis, maximum lesion size, number of resected metastasis, elevation of the tumor marker specific to the primary tumor, date of metastasectomy, other treatments, sites of recurrence after pulmonary metastasectomy and survival follow-up. The surgical indications and procedures were determined by each institution. Lymph node resection was not routinely performed and was at the discretion of each institution. Curability is defined as macroscopic and microscopic complete resection. DFI was calculated using the difference between the date of initial treatment for the primary tumor and the date of pulmonary metastasis diagnosis. Hospital mortality was defined as death within any time interval after an operation, if the patient had not been discharged from the hospital. Hospital-to-hospital transfer was not considered a discharge. Transfer to a nursing home or a rehabilitation unit was considered a hospital discharge unless the patient subsequently died of complications from the operation.
We investigated the surgical outcomes and prognostic factors of pneumonectomy. The date of first pulmonary resection was defined as the starting point. Postoperative follow-up was performed at the discretion of each institution.
Statistical analysis
Overall survival (OS) was analyzed using the Kaplan-Meier method. The Chi-square and Student’s t test were used to compare the percentages and mean values. Univariate and multivariate Cox proportional hazards regression analyses were used to evaluate potential prognostic factors. The data were analyzed using version 22 of the IBM SPSS software (IBM Corporation, Somers, NY, USA). A P value <0.05 was considered statistically significant.
Results
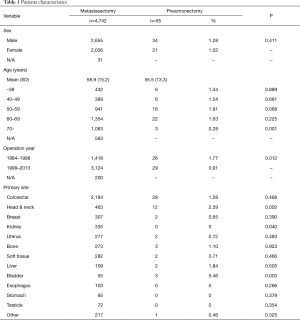
Of the 4,742 patients registered in the database, 55 patients (1.16%) were underwent pneumonectomy. Pneumonectomy were performed on 34 male patients (34/2,655, 1.28%) and 21 female patients (21/2,056, 1.02%); thus, there was no significant gender difference (P=0.411). The average age of pneumonectomy patients was 55.5±13.1 years old. The frequency of pneumonectomy on patients who were over 70 years (3/1,053, 0.28%) was lower (P=0.001). Further, the study showed a high tendency for pneumonectomy in patients who were in their 50 s (P=0.068). In the last 15 years, the frequency of pneumonectomy is significantly lower than in the previous 15 years (0.91% vs. 1.77%, P=0.012) (Table 1).
Full table
Primary tumors were found to be colorectal in 28 patients (28/2,184, 1.28%), head and neck in 12 patients (12/463, 2.59%), bone in three patients (3/273, 1.10%), bladder in three patients (3/55, 5.45%), and other regions in 9 patients (breast, uterus, liver, soft tissues in two patients, respectively, and pancreas in one patient). These results indicated that there was a high frequency of pneumonectomy in head and neck (P=0.002), and bladder (P=0.003) primary tumor patients. In contrast, no pneumonectomy was performed on kidney patients (0/335, P<0.05) (Table 1).
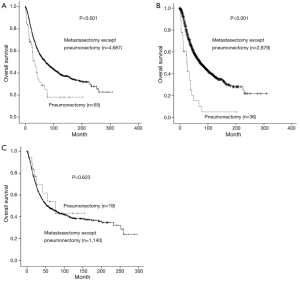
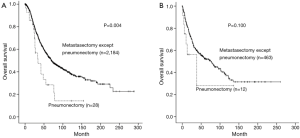
The average follows up period for patients who underwent pneumonectomy was 34.7 months, the number of patients surviving more than 5 and 10 years were 10 and 4, respectively. The 5-year survival rate for patients who underwent pneumonectomy was 28.9%, which was significantly lower (P<0.001) than that of the other metastasectomy, which had a rate of 53.4% (Figure 1A). It was shown that in patients 55 year or older, which is the mean age for pneumonectomy, there was a significant difference in 5-year survival rate between patients who underwent pneumonectomy and patients who underwent other metastasectomy (15.2% vs. 54.7%, P<0.001) (Figure 1B), whereas, such difference was not observed in patients under 55 years (54.0% vs. 49.0%, P=0.623) (Figure 1C). Regarding organ specificity in 5-year survival rate between patients who underwent pneumonectomy and patients who underwent other metastasectomy, there was a significant difference in colorectal patients (28.4% vs. 54.3%, P=0.004), but no significant difference in head and neck patients (28.1% vs. 52.6%, P=0.100) (Figure 2A,B).
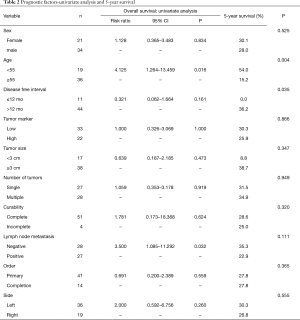
Five-year survival rates following pneumonectomy were 15.2% for patients 55 years or older and 54.0% for patients under 55 years (P=0.004). Further, analysis of DFI following the removal of primary tumor, showed that 5-year survival rates following pneumonectomy were 0% for DFI of 0 to 12 months and 38.8% for DFI over 12 months (P=0.035) (Table 2).
Full table
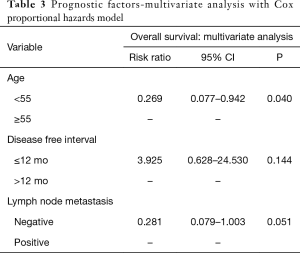
Univariate analysis of OS indicated that the age 55 year or older (P=0.016) and positive lymph node metastasis (P=0.032) were indicators of poor prognosis. Mediastinal or hilar lymph node dissection was performed on 48 patients, and mediastinal metastasis was found in 15 patients while hilar metastasis was found in 12 patients. However, gender, increased level of tumor markers, tumor size, numbers of metastasis, curability, and surgery site did not result in survival benefit (Table 2).
Multivariate analysis using age 55 or older, DFI of 0 to 12 months and lymph node metastasis, which are risk factors identified by survival rate and univariate analysis, indicated that age over 55 (P=0.040) was an independent risk factor (Table 3).
Full table
Among 55 patients who underwent pneumonectomy, there were 3 hospital mortalities (5.45%). These patients were all male in their 50s and had positive lymph node metastasis. One of the patients suffered cancer death 42 days after pneumonectomy, following the appearance of metastatic lesions in various regions of the body after an incomplete resection. The remaining two patients were an operative death on 23 days after surgery, and a sudden death due to unknown reason 35 days after surgery (Table 4).

Full table
Discussion
Metastatic lung tumors are caused by cancer cells that develop in other organs and travel through blood circulation to the lungs (7,8). Chemotherapy, a systemic therapy to treat advanced stage cancers, should be the choice for treating metastatic lung tumors; however, it is almost impossible to treat the tumor completely by chemotherapy alone, therefore, resection, a local therapy, is required (8,9). Metastatic lung tumors are often treated using a local therapy alone, but the therapeutic methods vary. Whether to perform surgery or chemotherapy first depends on the primary organ (9,10). At present, no standard therapeutic method to treat metastatic lung tumors has been established.
In 1997, the IRLMA published a report involving 5,206 patients and was the only large scale study conducted on metastatic lung tumors (7). In 1999, Koong published a report on pneumonectomy by subanalyzing the IRLMA report (5). Since then, other reports have been published that have analyzed between 200 and 1,200 patients at a single institution (1-4). This study was conducted by analyzing the data from 4,742 patients that had been collected over 30 years since 1984 at 25 institutions.
Historical details of the pneumonectomy for metastatic lung tumors were described by Koong in 1999 (5). Pneumonectomy was widely performed in the 1960s but became less popular after the 1970s. By the 1980s, pneumonectomy was performed in only 1% to 3% of metastatic lung tumor patients. According to this study, the frequency of performing pneumonectomy was low, having been performed in only 1.16% of all metastasectomy. In the last 15 years, the frequency of pneumonectomy is significantly lower than in the previous 15 years. We believe that due to technological advances of PET-CT and CT resolution, early phase cases increased. And, recently, there is a tendency to avoid pneumonectomy.
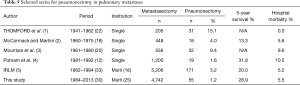
Compared with other surgery, such as lobectomy, pneumonectomy is frequently accompanied by complications; therefore, it is important to thoroughly evaluate the pros and cons of performing pneumonectomy. According to an annual summary published in 2010 by the Japanese Association for Thoracic Surgery, hospital mortality of patients who underwent pneumonectomy due to lung cancer was 3.0%, which was significantly higher compared with those who underwent lobectomy, which was 0.9% (11). Thomford reported that hospital mortality following pneumonectomy on patients with metastatic lung tumors was 0% (1); however, a different study reported that it was 5% to 10% (2-5), which was approximately the same proportion obtained in this study, which was 5.45% (Table 5).
Full table
On the other hand, 5-year survival rate for metastatic lung tumor patients following pneumonectomy is approximately 30%, indicating that there were patients who could be cured by complete resection, and, therefore, the pneumonectomy should not be completely disregarded. In this study, it was shown that the group consisting of patients under 55 years old with pneumonectomy showed approximately the same prognosis as patients who underwent other metastasectomy; therefore, it may be appropriate to consider the benefits of pneumonectomy in this younger age group. However, performing pneumonectomy should be carefully evaluated for patients who are elderly or DFI of 0 to 12 months. Further, patients with lymph node metastasis showed a poor prognosis.
One of the significant predictors of survival for treatment of pulmonary metastasis was the number of metastases (7,8). In pneumonectomy, the survival difference between single and multiple metastases did not reach statistical significance, due to the limited number of patient (5). Left side pneumonectomy had a better prognosis compared to right side pneumonectomy for lung cancer surgery (12), though there was no survival difference between left side and right side in this study, due to the limited number of patient.
This study was conducted using data from a registry of patients with metastatic lung tumor that was collected over 30 years at 25 institutions; therefore, there were several limitations to this study. Common reasons for deciding to perform pneumonectomy include the development of tumors at the hilar region, enlarged lymph nodes, or multiple nodules in one lung; however, the reasons for performing pneumonectomy in this study were unknown. The prognosis of 3 hospital mortalities was known, but the detailed prognosis of other patients who underwent pneumonectomy was difficult to obtain.
The IRLM reported pneumonectomy for metastatic lung tumors between 1962 and 1994, while this study reported those performed between 1984 and 2014, when the number of pneumonectomy is decreasing. It is believed that the number of pneumonectomy will further decrease as both radiation therapy and chemotherapy continue to progress. Given the lack of alternative therapy, selected patients with sufficient pulmonary reserve may be offered pneumonectomy.
Conclusions
Prior to making the decision to perform pneumonectomy, more careful consideration must be given to the patients 55 year or older and to patients with DFI of 0 to 12 months. Hospital mortality following pneumonectomy for metastatic lung tumor was an acceptable number.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Ethics Review Board (Teikyo University Review Board 10-108) and other institutions, and waived the need for informed consent from patients, as long as patient data remained anonymous.
References
- Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965;49:357-63. [PubMed]
- McCormack PM, Martini N. The changing role of surgery for pulmonary metastases. Ann Thorac Surg 1979;28:139-45. [Crossref] [PubMed]
- Mountain CF, McMurtrey MJ, Hermes KE. Surgery for pulmonary metastasis: a 20-year experience. Ann Thorac Surg 1984;38:323-30. [Crossref] [PubMed]
- Putnam JB Jr, Suell DM, Natarajan G, et al. Extended resection of pulmonary metastases: is the risk justified? Ann Thorac Surg 1993;55:1440-6. [Crossref] [PubMed]
- Koong HN, Pastorino U, Ginsberg RJ. Is there a role for pneumonectomy in pulmonary metastases? International registry of lung metastases. Ann Thorac Surg 1999;68:2039-43. [Crossref] [PubMed]
- Shiono S, Sato T, Horio H, et al. Outcomes and prognostic factors of survival after pulmonary resection for metastatic gastric cancer. Eur J Cardiothorac Surg 2013;43:e13-6. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. International registry of lung metastases. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Monteiro A, Arce N, Bernardo J, et al. Surgical resection of lung metastases from epithelial tumors. Ann Thorac Surg 2004;77:431-7. [Crossref] [PubMed]
- Rusch VW. Pulmonary metastasectomy. Current indications. Chest 1995;107:322S-31S. [Crossref] [PubMed]
- van Geel AN, Pastorino U, Jauch KW, et al. Surgical treatment of lung metastases: the European organization for research and treatment of cancer-soft tissue and bone sarcoma group study of 255 patients. Cancer 1996;77:675-82. [Crossref] [PubMed]
- Kuwano H, Amano J, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2010: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2012;60:680-708. [Crossref] [PubMed]
- Gu C, Wang R, Pan X, et al. Comprehensive study of prognostic risk factors of patients underwent pneumonectomy. J Cancer 2017;8:2097-103. [Crossref] [PubMed]


