Unusual cardiac paraganglioma mimicking an atypical carcinoid tumor of the lung
Introduction
Cardiac paraganglioma (PG) are a rare type of chromaffin cell tumor. Chromaffin cell tumors occur at a rate of only 1.5–9 per million people and most commonly occur in the adrenal gland as a pheochromocytoma (1). However, when they occur outside the adrenal gland they are called PG and may occur at various points along sympathetic or parasympathetic ganglia. These tumors can be functional if the cells actively secrete catecholamines or non-functional if the tumor cells are non-secreting. Only 2% of all chromaffin cell tumors occur as thoracic PG (2). Thoracic PG can be in the posterior mediastinum arising from para-aortic sympathetic ganglia or less commonly in the middle mediastinum, arising from cardiac structures themselves, which are appropriately designated as cardiac PG. Cardiac PG usually originate from the left atrium and less commonly arise from the aortic root. Rarer still, very few cardiac PGs have been reported to arise from other cardiac chambers such as the right atrium or left ventricle (3,4).
Cardiac PG are typically benign and are best treated by surgical resection. This is most often achieved through median sternotomy with cardiopulmonary bypass. We present an unusual case of a cardiac PG originally misdiagnosed as an atypical carcinoid tumor that was successfully resected through a right thoracotomy, without cardiopulmonary bypass.
Case presentation
A 64-year-old woman with asthma, hypertension, and gastroesophageal reflux disease (GERD) initially presented to another hospital with persistent cough and back pain. Computed tomography (CT) imaging revealed a 4 cm × 3 cm right hilar mass abutting the superior vena cava (SVC) and right pulmonary artery and vein. CT guided biopsy of the mass revealed nests of polygonal cells with prominent nuclear pleomorphism concerning for an atypical carcinoid neuroendocrine tumor. This diagnosis was upheld by positive staining for chromogranin A and synaptophysin. Two weeks later, surgical excision of the right hilar mass was attempted with a right sided thoracotomy. However, the procedure was aborted due to excessive bleeding. The next day, right internal mammary artery embolization was performed and follow-up CT angiogram showed some devascularization of the mass, but still with persistent blush. The patient was then transferred to our institution for further care.
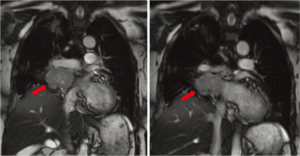
Her preoperative work-up included magnetic resonance angiography (MRA), pulmonary perfusion studies, and a full body octreotide scan. MRA of the chest showed a mass abutting the right atrium, left atrium, right main pulmonary artery, SVC, and right pulmonary veins with no evidence of direct invasion of these structures (Figure 1). Pulmonary perfusion study showed that her right lung contributed less than a third of pulmonary function, indicating that pneumonectomy would be possible. Octreotide scan demonstrated a solitary mass in the right hilar region with no metastases. Urine or plasma catecholamine levels were not obtained during this period. She had excellent oxygen saturation on room air and was discharged home in stable condition to await the scheduled procedure.
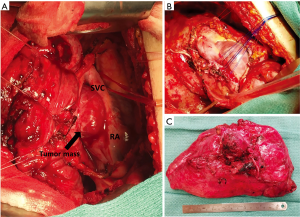
Intra-operative course
The patient returned one week later for a right pneumonectomy and right hilar mass excision through a right sided thoracotomy with cardiopulmonary bypass on standby. The previous right thoracotomy incision was re-opened and the thoracic cavity was entered through the fifth intercostal space. Dense adhesions throughout the right thorax were taken down and the dissection was continued down towards the hilum. The tumor was noted to have intimate mediastinal involvement with suspicion that the tumor itself arose from the wall of the atrium rather than the lung parenchyma. The pericardium was opened at the level of the right atrium which provided excellent visualization of the tumor (Figure 2A). The tumor was highly vascular as it was separated from the SVC, right atrium, and right main pulmonary artery. The right main pulmonary artery was then controlled and snared, which did not affect bleeding from the dissection. The dissection was continued to the wall of the left atrium and the tumor was noted to be attached to the left atrial wall with the majority of the tumor’s vascular supply originating from this area. The portion of left atrium associated with the tumor was plicated with pledgeted sutures to allow separation of the tumor without hemorrhage, which was completed successfully (Figure 2B). Surrounding tissue specimens and lymph nodes were sent for frozen section to pathology which confirmed negative margins. The dissection was continued posteriorly and the tumor was noted to have some involvement with the right superior and inferior pulmonary veins. The right pneumonectomy was completed with tumor intact and sent to pathology for examination (Figure 2C). The wound bed was irrigated, hemostasis ensured, suture lines were coated with ProGEL pleural air leak sealant (Neomend, Inc., Irvine, CA, USA), and the pericardium was repaired with 0.1 mm Gore-Tex patch. The patient was hemodynamically stable and was extubated in the operating room. Estimated blood loss was 600 cc and the patient received two units of packed red blood cells intraoperatively.
Histopathological analysis
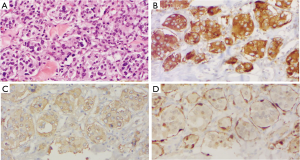
The gross tissue specimen received by pathology consisted of a 293.38-gram right lung with a 4.3 cm × 2.9 cm × 2.2 cm, tan-to-dark brown, hemorrhagic mass in the hilum. The tumor was well-circumscribed with no infiltration into the adjacent lung parenchyma. Cross sections revealed that the mass was adherent to the right pleura with extensive embolization effects. Histological examination of the tumor demonstrated Zellballen-like organoid nests of eosinophilic, polygonal chief cells containing prominent nuclear pleomorphism, surrounded by dense fibrovascular stroma. Focal lymphovascular invasion was present. At immunohistochemical staining, the cell populations were diffusely positive for chromogranin A and synaptophysin. The protein S100 highlighted the neoplasm diffusely, with increased intensity inside individual cells that corresponded to sustentacular cells (Figure 3). Increased mitotic activity was also appreciated, with approximately greater than 10% of cells staining positive for Ki67. Notably, cytokeratin 7 and TTF-1 staining was negative. The definitive diagnosis was PG of the mediastinum, and all bronchial and vascular resection margins were negative for malignancy. Furthermore, one Station 4R lymph node, two Station 7 lymph nodes, and nine peribronchial lymph nodes were free of metastasis.
Discussion
The present case of cardiac PG is an example of rare PG in that it specifically originated from a cardiac structure, with such tumors being reported to occur in the thorax in only 1–2% of all PG (2). PGs are typically benign and the delineation of malignancy is based on metastasis to other areas. The majority of PG are sporadic compared to less commonly found familial germline mutations. Malignancy has been reported as high as 50% in familial types when the primary tumor was located in the abdomen or head and neck, but no cardiac PG were included in this finding (5). The rate of malignancy in cardiac PG remains unclear. Up to 17 genetic mutations have been identified and mutations in genes for succinate dehydrogenase have recently been shown to have a strong connection to familial types of cardiac PG (6,7). Therefore, genetic testing is recommended in patients with cardiac PG (8).
Presentation
The clinical presentation of cardiac PG differs based on the functional status of the tumor. Presenting symptoms of functional cardiac PG are well reported with key symptoms such as hypertension, palpitations, headache, and sweating being most prevalent (9). Non-functional PG are much more subtle in clinical presentation and are typically asymptomatic until the tumor compromises cardiac function due to local mass effect. Several reported cases have illustrated this effect, describing tumors obstructing the left-ventricular outflow tract or even directly occluding coronary artery blood flow (10). In these instances, presenting symptoms included chest pain, dyspnea on exertion, fatigue, and cardiac murmur on physical exam. The tumors were then detected on subsequent cardiac angiography or other imaging during clinical work-up of these symptoms (11,12). Local mass effect from tumors on nearby respiratory system structures may also cause pulmonary symptoms such as cough, hemoptysis, and stridor. Similarly, mass effect on the esophagus may present as dysphagia. In our case, the initial clinical presentation was primarily pulmonary symptoms of chronic cough due to tumor compression of the airways of the right bronchial tree and associated pulmonary vasculature. Although imaging did note external compression of both atrial structures, the tumor did not affect coronary blood flow or cardiac output and therefore did not produce typical cardiovascular clinical symptoms.
Diagnosis
Cardiac PG are also diagnosed differently based on the functional status of the tumor. Functional cardiac PG may be diagnosed with a combination of imaging and biochemical analysis of blood or urine for significantly increased metabolites. Specifically, urine fractionated catecholamine and metanephrine levels are useful in identifying secreting PG (9). Non-functional PG may be suspected due to hypervascularity of identified tumors on imaging. Cardiac PG are extremely vascular and are frequently supplied by branches of the coronary arteries. Therefore, cardiac angiography often provides detailed visualization of these hypervascularized masses which is useful for diagnosis and surgical planning. In addition to angiography, multiple imaging modalities such as CT, magnetic resonance imaging (MRI), iodine-131-meta-iodobenzylguanidine (MIBG) scintigraphy, octreotide scintigraphy, and transesophageal echocardiography (TEE) have been reported to be useful in diagnosis and localization of cardiac PG (10). Unfortunately, none of the above imaging modalities are able to provide definitive diagnosis. Radiographically, PG may be mistaken for other neuroendocrine tumors such as small cell lung cancer or carcinoid (13).
Definitive diagnosis remains histopathological examination. The pathologists at the outside hospital to which our patient presented initially designated her tumor as an atypical carcinoid. This misclassification is surprisingly common when considering the differential diagnosis of PG, even in the modern era of immunohistochemical staining. In fact, PGs and atypical carcinoids are similar-appearing neuroendocrine tumors. The sustentacular cells of PG are frequently difficult to identify with hematoxylin and eosin, and the characteristic Zellballen organoid growth pattern is not always diagnostic. Even electron microscopy can fail to differentiate the tumors, as each contains neurosecretory granules (14). Ultimately, immunohistochemistry is essential for accurate diagnosis: PGs are generally negative for cytokeratin, calcitonin, carcinoembryonic antigen, epithelial membrane antigen, and often bombesin. S100 protein and glial fibrillary acidic protein are also important for visualizing PG sustentacular cells. However, the absence or presence of positive staining does not always accurately identify either tumor. For example, cytokeratin positivity has been observed in a limited number of PGs of the head and neck and cauda equina, while other cases of atypical carcinoid have failed to stain for cytokeratin (14-16). Therefore, accurate histopathological analysis of PG with immunohistochemistry as an adjunct to light microscopy is essential for diagnosis due to the nebulous clinical presentation and lack of symptoms often associated with these tumors.
Treatment
The standard treatment for cardiac PG is complete surgical resection. However, surgical excision of these tumors is challenging due to their intimate connection with surrounding vital structures, hypervascularity with increased risk of severe hemorrhage, and difficulty in accessing the tumor. The most common surgical approach to resect cardiac PG is median sternotomy with the use of cardiopulmonary bypass. An extensive literature review of cardiac PG from 1974–2014 by Wang et al. indicated that the majority of the cases were resected through this approach. They also reported 15 cases being resected through a thoracotomy incision and only four cases not requiring the use of cardiopulmonary bypass (17). One case report indicated an attempted resection of a cardiac PG arising from the left atrium by thoracotomy, but converted to median sternotomy intraoperatively (18). Another report utilizing a thoracotomy approach resulted in severe bleeding leading to intraoperative cardiac arrest. The authors of this case recommended use of cardiopulmonary bypass as the main takeaway from their case (19). The case presented by the authors is rare in the fact that the tumor was successfully resected through a thoracotomy incision and although cardiopulmonary bypass was available on standby throughout the procedure, it was not required.
Due to the hypervascular nature of cardiac PG, some attention has been given to preoperative arterial embolization to minimize intraoperative bleeding. In our case, the patient received right internal mammary artery embolization after the first unsuccessful attempt at tumor resection. In the review of seven cardiac PGs by Ramlawi et al., one patient received preoperative arterial embolization and the authors acknowledge the utility of preoperative arterial embolization in avoiding massive intraoperative hemorrhage from previous experience at their institution (20). Additionally, one other case report described successful embolization of a large vessel of the left coronary artery found to be supplying a large intrapericardial PG (21).
Outcomes
Surgical resection of cardiac PG is considered a high-risk procedure due to increased risk of intraoperative hemorrhage from the highly vascularized nature of these tumors and frequent extensive adhesions to surrounding vital structures. Intraoperative mortality has been reported to be 3.1% by Wang et al.’s review of 158 cases of cardiac PGs (17). An additional 2.5% mortality was reported for the immediate postoperative course. A review of individual case reports from 2014–2016, two years since Wang et al.’s review, shows no intraoperative mortality reported from 11 cases (12,18,19,22-27). Among these cases, one instance of intraoperative cardiac arrest occurred due to severe hemorrhage and one unexpected intraoperative circulatory collapse, however both ended with successful outcomes (24,26).
After recovery from surgery, benign cardiac PGs that are completely resected with negative margins have been associated with favorable outcomes in survival with low rates of tumor recurrence (18,26). For those surviving the immediate post-operative course, a 1-year survival of 98.2% and 5-year survival of 78.2% has been reported (17).
Conclusions
The present case of cardiac PG provides important contributions to the current literature of these rare tumors by highlighting the proper approach to diagnosing PG vs. atypical carcinoid, the preoperative management with potential arterial embolization, and describing surgical technique that achieved complete resection through thoracotomy without the use of cardiopulmonary bypass.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written consent for publication was unable to be given by the patient, who expired before this report was written. This report is important to the medical and surgical community by aiding in the correct diagnosis of a rare and challenging clinical condition. A reasonable individual would be unlikely to object to publication. Consent would be unusually burdensome to obtain.
References
- Joynt KE, Moslehi JJ, Baughman KL. Paragangliomas: etiology, presentation, and management. Cardiol Rev 2009;17:159-64. [Crossref] [PubMed]
- Orringer MB, Sisson JC, Glazer G, et al. Surgical treatment of cardiac pheochromocytomas. J Thorac Cardiovasc Surg 1985;89:753-7. [PubMed]
- Mandak JS, Benoit CH, Starkey RH, et al. Echocardiography in the evaluation of cardiac pheochromocytoma. Am Heart J 1996;132:1063-6. [Crossref] [PubMed]
- Lorusso R, De Cicco G, Tironi A, et al. Giant primary paraganglioma of the left ventricle. J Thorac Cardiovasc Surg 2009;137:499-500. [Crossref] [PubMed]
- Brouwers FM, Eisenhofer G, Tao JJ, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab 2006;91:4505-9. [Crossref] [PubMed]
- Martucci VL, Emaminia A, del Rivero J, et al. Succinate dehydrogenase gene mutations in cardiac paragangliomas. Am J Cardiol 2015;115:1753-9. [Crossref] [PubMed]
- Baysal BE. On the association of succinate dehydrogenase mutations with hereditary paraganglioma. Trends Endocrinol Metab 2003;14:453-9. [Crossref] [PubMed]
- Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001;69:49-54. [Crossref] [PubMed]
- Brown ML, Zayas GE, Abel MD, et al. Mediastinal paragangliomas: the Mayo Clinic experience. Ann Thorac Surg 2008;86:946-51. [Crossref] [PubMed]
- Hayek ER, Hughes MM, Speakman ED, et al. Cardiac paraganglioma presenting with acute myocardial infarction and stroke. Ann Thorac Surg 2007;83:1882-4. [Crossref] [PubMed]
- Marinho B, Malangatana G, Almeida J, et al. Acute coronary syndrome as clinical presentation of cardiac paraganglioma. Rev Port Cir Cardiotorac Vasc 2011;18:153-5. [PubMed]
- Steger CM, Laufer G, Moser PL, et al. Incidental finding of a cardiac paraganglioma. Pathologe 2015;36:389-93. [Crossref] [PubMed]
- Ferlito A, Milroy CM, Wenig BM, et al. Laryngeal paraganglioma versus atypical carcinoid tumor. Ann Otol Rhinol Laryngol 1995;104:78-83. [Crossref] [PubMed]
- Orrell JM, Hales SA. Paragangliomas of the cauda equina have a distinctive cytokeratin immunophenotype. Histopathology 1992;21:479-81. [Crossref] [PubMed]
- Wenig BM, Hyams VJ, Heffner DK. Moderately differentiated neuroendocrine carcinoma of the larynx. A clinicopathologic study of 54 cases. Cancer 1988;62:2658-76. [Crossref] [PubMed]
- Johnson TL, Zarbo RJ, Lloyd RV, et al. Paragangliomas of the head and neck: immunohistochemical neuroendocrine and intermediate filament typing. Mod Pathol 1988;1:216-23. [PubMed]
- Wang JG, Han J, Jiang T, et al. Cardiac paragangliomas. J Card Surg 2015;30:55-60. [Crossref] [PubMed]
- El-Ashry AA, Cerfolio RJ, Singh SP, et al. Cardiac paraganglioma. J Card Surg 2015;30:135-9. [Crossref] [PubMed]
- Yamamoto Y, Kodama K, Yamato H, et al. Successful Removal of Giant Intrapericardial Paraganglioma via Posterolateral Thoracotomy. Case Rep Surg 2014;2014:308462. [Crossref] [PubMed]
- Ramlawi B, David EA, Kim MP, et al. Contemporary surgical management of cardiac paragangliomas. Ann Thorac Surg 2012;93:1972-6. [Crossref] [PubMed]
- Brichon PY, Chavanon O, Thony F, et al. An intrapericardial paraganglioma with embolization of a large vessel from the left coronary artery. Eur J Cardiothorac Surg 2014;45:e234. [Crossref] [PubMed]
- Saththasivam P, Herrera E, Jabbari OA, et al. Cardiac Paraganglioma Resection With Ensuing Left Main Coronary Artery Compromise. J Cardiothorac Vasc Anesth 2017;31:236-9. [Crossref] [PubMed]
- Del Forno B, Zingaro C, Di Palma E, et al. Cardiac Paraganglioma Arising From the Right Atrioventricular Groove in a Paraganglioma-Pheochromocytoma Family Syndrome With Evidence of SDHB Gene Mutation: An Unusual Presentation. Ann Thorac Surg 2016;102:e215-6. [Crossref] [PubMed]
- Hui S, Miao Q, Luo A, et al. Unexpected Circulatory Collapse After Cardiac Paraganglioma Resection: Rescue With Intra-Aortic Balloon Pump and Extracorporeal Membrane Oxygenator. J Cardiothorac Vasc Anesth 2016;30:1057-60. [Crossref] [PubMed]
- González-Santos JM, Arnáiz-García ME, Muñoz-Herrera Á, et al. Mediastinal paraganglioma fed by the left circumflex artery. Interact Cardiovasc Thorac Surg 2016;23:835-6. [Crossref] [PubMed]
- Yadav PK, Baquero GA, Malysz J, et al. Cardiac paraganglioma. Circ Cardiovasc Interv 2014;7:851-6. [Crossref] [PubMed]
- Irqsusi M, Vogt S, Rexin P, et al. Extraadrenal biatrial cardiac paraganglioma: diagnosis, histological criteria and surgical management. Ann Thorac Surg 2014;97:2200. [Crossref] [PubMed]


