Sarcopenia is a predictor of outcomes after lobectomy
Introduction
Lung cancer is the second most commonly diagnosed cancer in the US, and is the leading cause of cancer related mortality (1). Low dose CT screening has been shown to result in decreased disease related mortality due in part to diagnosis of lung cancer at an earlier stage when it is potentially curable (2). For patients able to tolerate an operation, lobectomy is the operation preferred for resection of early stage lung cancer (3,4), although for Stage IA lung cancer, sublobar resection may be an equivalent option (5). Lobectomy is, however, associated with operative morbidity and mortality (6,7), although surgical volume and advances in operative technique and post-operative care have led to a decline in postoperative complication rates (4). The ability to accurately predict postoperative morbidity and mortality is increasingly important as the incidence of early stage lung cancer will likely continue to rise with the aforementioned screening guidelines and other options other than a lobectomy exist for the patient at a high risk. With an increasingly aging population seeking consultation for surgery, tools to predict peri-operative outcomes have become ever important.
Factors that have been associated with worse peri-operative outcomes following lobectomy include increasing age, male sex, smoking history, comorbidities (including COPD and prior cardiovascular event), degree of dyspnea, type of operation (thoracotomy versus thoracoscopy), right-sided tumors, decreased facility surgical volume, anemia, dysnatremia, and functional status (6-10) as assessed by spirometry, exercise testing to determine peak VO2, performance status, and evaluation of physical reserve (11). Frailty, defined as a lack of physiologic reserve resulting in adverse outcomes, has also been recognized as an independent risk factor for inferior outcome in regard to mortality and morbidity in the elderly, those undergoing surgery, and cancer patients (12-14). The phenotype of physical frailty is associated with decreased muscle mass, and sarcopenia has been associated with poor short and long-term outcomes, even in overweight and obese patients (15-18). CT imaging has been found to correlate well with other methods of evaluating muscle mass, which is useful as CT imaging is routinely done prior to resection (19). In lung cancer, recent studies have found evidence that sarcopenia is an independent risk factor for decreased overall survival (20,21). In those studies, assessment of muscle mass was done at the level of L3, which sometimes necessitates a CT scan lower than routinely performed to evaluate chest pathology. Muscle mass at T12 has been found to correlate well with muscle mass at L3, and thus pre-operative assessment may not need to include additional imaging of the abdomen to be able to accurately estimate muscle mass (22). In COPD, decreased cross sectional area, as measured on CT scans, of the pectoralis muscles (CSA-PM) and of the erector spinae muscles (CSA-ESM) at T12 have been found to correlate with survival, symptoms, and physiologic function (23,24). It is unclear if such an association exists in patients undergoing surgical resection. We hypothesized that there is an association between muscle mass and short-term postoperative morbidity and mortality for lobectomy and examined this association using our institutional database.
Methods
Study subjects and measurements
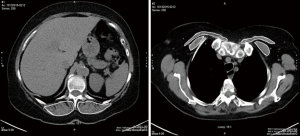
This study was approved by our Institutional Review Board (BDR-073016). All lobectomy or bilobectomy procedures performed between January 2014 and December 2015 at our institution were retrospectively reviewed. Demographic data, clinical information, interventions, length of stay (LOS) in the hospital or intensive care unit (ICU), and procedure related complications were collected in an institutional prospective database. Before the procedure, all patients underwent physical examination, pulmonary function testing, blood tests, chest computed tomography (CT) or positron emission tomography (PET-CT). The CSA-ESM and CSA-PM were measured using the AW Server 3.2 Workstation 3D volume viewer with free hand region of interest (General Electric Healthcare Inc., Chicago USA). The volume of skeletal muscles was estimated using a cross sectional area on a single-slice axial CT scans. The measurements for the ESM and PM were performed bilaterally at the level of thoracic spine 12 (T12) and within 1 centimeter of the sterno-clavicular joint, respectively (Figure 1). As absolute skeletal muscle mass is correlated with height, the cross-sectional areas were then normalized for height (cm2/m2) (25), and listed as height adjusted ESM (HA-ESM) and height adjusted PM (HA-PM).
Statistical analysis
Patient demographics, clinical characteristics, and cross-sectional area adjusted for height were reported for the overall sample and by gender using means and standard deviations (SD) for continuous data, and frequencies and relative frequencies for categorical data.
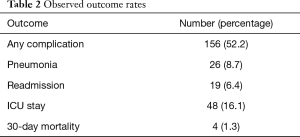
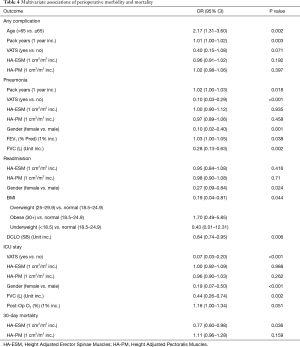
Morbidity (general complication, pneumonia, readmission, and ICU stay) and mortality (with 30-days) outcomes were summarized for the overall sample using frequencies and relative frequencies; with rates estimated using 95% CI obtained from Jeffrey’s prior method. The association between these outcomes and quantitative height adjusted cross-sectional areas were evaluated using logistic regression models. The models were fit using Firth’s penalized function and odds ratios, with corresponding 95% confidence intervals, were obtained from the model estimates. A covariate adjusted analysis was conducted using multi-variable logistic regression, where the additional demographic and clinical covariates were chosen using a backwards selection method (alpha exit =0.1).
The length of hospital stay (LOS) was summarized using the mean and standard deviation; and graphically using a histogram. A 95% CI about the mean LOS was obtained using standard methods. The association between LOS and the muscle measurements was assessed using scatter plots and Pearson’s correlation coefficient. A covariate adjusted analysis was conducted using a linear regression model, where additional demographic and clinical covariates are chosen using the backwards selection method (alpha exit =0.1). All model assumptions were verified graphically and a log-transformation was applied. The correlation of HA-ESM and HA-PM was explored using a scatter plot and Pearson’s correlation coefficient. All analyses were conducted in SAS v9.4 (Cary, NC) at a significance level of 0.05.
Results
A total of 299 patients underwent lobectomy between January 2014 and December 2015. There were 186 females (62.2%) with a mean age of 67.1±11.4 years and 113 males (37.8%) with a mean age of 68.1±9.4 years. Most patients were either overweight (115 patients, 38.5%) or obese (106 patients, 35.5%), with 74 being of normal weight (24.7%) and only 4 patients (1.3%) were underweight. A total of 121 patients were American Society of Anesthesiologists (ASA) class II (40.6%), with the remaining 177 patients being ASA class III/IV (59.4%). All patients were current or former smokers with a mean smoking history of 38 pack-years. Of the 299 procedures, 278 (93%) were performed by video assisted thoracoscopic surgery (VATS). Except for two cases with benign diagnoses of aspergillus infection and foreign body aspiration, all other patients were diagnosed with malignant pathology. Two hundred and eighty-three out of the 299 patients (94.6%) were diagnosed with lung cancers including small and non-small cell lung cancers, large cell carcinoma and carcinoid tumors, while the rest were tumors metastatic to the lung. Patient characteristics are summarized in Table 1.

Full table
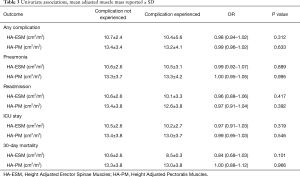
The overall complication rate was 52.2%. The majority of the complications were minor, such as post-operative atelectasis. The overall pneumonia rate was 8.7% and the 30-day mortality rate was 1.3% (Table 2). Multivariate analysis demonstrated a significant increase in complication rates for those older than 65, those who underwent thoracotomy as opposed to VATS, and as pack-year history of smoking increased. VATS procedures were associated with a lower pneumonia rate. Lower DLCO was associated with a higher rate of readmission. Female sex was associated with a higher rate of pneumonia, higher rate of readmission, and an increase in the ICU length of stay (Table 3).
Full table
Full table
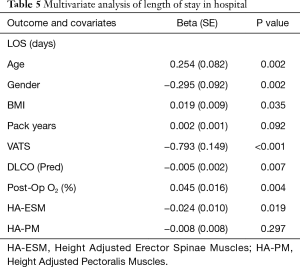
The means of HA-ESM and HA-PM were 10.6±2.6 and 13.3±3.8 cm2/m2. HA-ESM and HA-PM muscle measurements were significantly positively correlated (P<0.001). HA-ESM, and HA-PM were not found to have a statistically significant association with general complication rate, rates of pneumonia, readmission, or ICU length of stay in univariate or multivariate analysis (Tables 3,4). When adjusting for other factors, as HA-ESM increases the 30-day mortality was significantly found to decrease, OR 0.77 (95% CI, 0.60–0.98, P=0.036). There was no significant association between HA-PM with 30-day mortality. The mean LOS for patients having lobectomy was 7.0 days (95% CI, 5.5–8.4 days). As there was right skew with a small number of admissions causing the mean LOS to be greater than the median LOS, log-LOS was used for analysis to provide a more symmetric distribution. Multivariate analysis demonstrated a significant inverse association between log-LOS and HA-ESM (P=0.019) (Table 5).
Full table
Full table
Discussion
Lobectomy remains the preferred treatment for early stage lung cancer, with sublobar resections such as segmentectomy or wedge resection also an option for small peripheral lesions or in those with operative risk (4,5,8). For patients unable or unwilling to undergo surgical resection, stereotactic body radiotherapy can be done, although outcomes with lobectomy have mostly been found to be superior (26,27). Should the incidence of early stage lung cancer rise as might be expected with appropriate screening, the ability to predict complications pre-operatively will be of high clinical utility so that it may be determined if a patient would be better served with sublobar resection or a non-surgical treatment option. Our results show that HA-ESM is significantly inversely correlated with 30-day mortality and length of stay; HA-PM was not significantly associated with post-lobectomy morbidity or mortality. The addition of these metrics may help the decision-making process prior to intervention.
Efforts to define preoperative risk factors have increasingly focused on patient characteristics beyond standard evaluation of pulmonary reserve and cardiac risk factors. As the population ages, much research has been done to assess frailty and its relation to outcomes, and sarcopenia has been an increasing topic of interest. In the past, BMI has been used as a marker of nutritional status and function, but as rates of obesity continue to climb, muscle depletion has been found to exist independent from BMI and is associated with poor outcomes. A number of studies describe this relationship in non-postoperative patients. Srikanthan et al. noted that sarcopenia can predict mortality, as this was lower in all participants of a cohort of older adults, defined as >55 years old for males and >65 years old for females, with the most muscle mass as compared with those with the least muscle mass, independent of BMI (17). In that study, assessment of muscle mass was done via bioelectrical impedance, with a muscle mass index calculated as muscle mass divided by height squared. Mourtzakis et al. noted that CT imaging of the muscles at the L3 level to assess for muscle mass was equivalent to dual-energy X-ray absorptiometry and superior to bioelectrical impedance analysis (19). Subsequently, L3 muscle index (L3MI) has been used to assess for sarcopenia. Martin et al. found that in patients with lung and GI cancer, CT imaging revealed otherwise occult muscle depletion and that inferior survival was related to both a traditional definition of involuntary weight loss of cachexia and muscle depletion at the level of L3 (16). Choi et al. found that L3MI predicted poor overall survival in patients with pancreatic cancer, with an interesting finding that patients who had a fall in BMI but with preserved muscle mass did not have a deleterious impact on survival (15). Thus, the use of measuring the surface area of musculature adjusted for height to assess for sarcopenia has been well validated and meaningful for patient outcomes.
This has held true for patients undergoing surgery, as sarcopenia has been found to be an independent factor for morbidity and mortality in addition to other well-recognized risk factors such as comorbidities. Du et al. found that in a cohort of elderly patients undergoing emergent surgery sarcopenia was significantly associated with postoperative complications, and in-hospital mortality (12). Kudou et al. evaluated postoperative outcomes in patients undergoing surgical resection for esophagogastric or upper gastric cancer, and those patients with sarcopenia had inferior overall survival and recurrence-free survival (28). Zhou et al. found that sarcopenia in patients undergoing radical gastrectomy was an independent risk factor for postoperative complications (18). Hervochon et al. demonstrated that BMI and total psoas area at the level of L3 were independent prognostic factors for patients undergoing pneumonectomy for NSCLC (29). Suzuki et al. examined 90 consecutive patients undergoing lung resection for stage I NSCLC, and using L3MI to assess for sarcopenia, found that sarcopenia was an independent prognostic factor for survival as patient with a low muscle mass had a significant decrease in 5-year overall survival (20). Tsukioka et al. reported on 215 male patients with pathological stage I NSCLC, and used a calculated L3MI derived from an equation using BSA to define sarcopenia; in their report, sarcopenia did not affect short-term outcomes, but was associated with a shorter median overall survival (21).
The majority of studies examining CT imaging for assessment of sarcopenia have involved measurement of muscle cross sectional area at the level of the lumbar spine, requiring abdominal imaging. For patients undergoing thoracic procedures, imaging of the abdomen is not always routinely done, and utilizing existing imaging of the thorax would be more practical, assuming that muscle mass at the level of L3 and within the thorax is comparable. Nemec et al. recently compared skeletal muscle indices at the levels of L3, T12, and T7, finding that L3MI and T12 muscle index (T12MI) were significantly correlated, whereas the correlation of the muscle indices at L3 and T7 were not as strongly correlated (22). In addition, investigators have now used imaging of the thorax to demonstrate that thoracic muscle mass is associated to morbidity and mortality. McDonald et al. found that CT-derived pectoralis muscle area was more predictive of COPD related traits such as spirometric measures, dyspnea, and function than BMI (23). Tanimura et al. measured CSA-PM and CSA-ESM at T12 in patients with COPD, with CSA-ESM being found to be a risk factor for mortality (24).
We chose to look at the erector spinae muscles and the pectoral muscles because of the correlation with outcomes in chronic lung disease and due to their ready availability on imaging that was already obtained as part of our standard pre-operative assessment. An alternative approach would have been to examine all skeletal muscle at the level of T12 to obtain a T12 muscle index similar to what Nemec et al. described, although the erector spinae muscles, as antigravity muscles, reflect physical activity more than other muscle groups (30).
A limitation of our study is its retrospective nature. We also have a high proportion of our patients undergoing VATS lobectomy as opposed to thoracotomy, so we cannot draw a conclusion as to what the experience may be in centers that perform more thoracotomies for lobectomy. We did not define a cutoff for sarcopenia or separate the patients into quartiles as previous authors have done, but we thought that the use of muscle mass as a continuous variable made clinical sense. In the future, if assessment of sarcopenia becomes a standard addition to pre-operative testing, a more standardized definition, such as T12MI, may be useful risk-stratify patients. Future work would include looking at larger population of patients undergoing a variety of procedures, and to define strategies that might ameliorate post-lobectomy complications in patients who are found to have sarcopenia in the preoperative period, and to compare outcomes if patients with sarcopenia undergo sublobar resection or another treatment modality instead of lobectomy.
Conclusions
CT imaging of the chest allows for measurement of the cross-sectional area of muscle at the level of T12, and HA-ESM is associated with hospital length of stay and 30-day mortality following lobectomy. We did not find any association of HA-PM with post-lobectomy morbidity or mortality. Determination of the cross-sectional area of muscle at the level of T12 may be clinically useful for pre-operative evaluation and risk stratification.
Acknowledgements
Funding: This work was supported by Roswell Park Cancer Institute and National Cancer Institute (NCI) grant P30CA016056.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by our Institutional Review Board (BDR-073016).
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest 2012;142:1620-35. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; discussion 62-4. [Crossref] [PubMed]
- Dales RE, Dionne G, Leech JA, et al. Preoperative prediction of pulmonary complications following thoracic surgery. Chest 1993;104:155-9. [Crossref] [PubMed]
- Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg 2009;88:1093-9. [Crossref] [PubMed]
- Gulack BC, Yang CJ, Speicher PJ, et al. A Risk Score to Assist Selecting Lobectomy Versus Sublobar Resection for Early Stage Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1814-20. [Crossref] [PubMed]
- Jean RA, DeLuzio MR, Kraev AI, et al. Analyzing Risk Factors for Morbidity and Mortality after Lung Resection for Lung Cancer Using the NSQIP Database. J Am Coll Surg 2016;222:992-1000.e1. [Crossref] [PubMed]
- Rosen JE, Hancock JG, Kim AW, et al. Predictors of mortality after surgical management of lung cancer in the National Cancer Database. Ann Thorac Surg 2014;98:1953-60. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e90S.
- Du Y, Karvellas CJ, Baracos V, et al. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery 2014;156:521-7. [Crossref] [PubMed]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. [Crossref] [PubMed]
- Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015;26:1091-101. [Crossref] [PubMed]
- Choi Y, Oh DY, Kim TY, et al. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PLoS One 2015;10:e0139749. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Srikanthan P, Karlamangla AS. Muscle Mass Index As a Predictor of Longevity in Older Adults. Am J Med 2014;127:547-53. [Crossref] [PubMed]
- Zhou CJ, Zhang FM, Zhang FY, et al. Sarcopenia: a new predictor of postoperative complications for elderly gastric cancer patients who underwent radical gastrectomy. J Surg Res 2017;211:137-46. [Crossref] [PubMed]
- Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997-1006. [Crossref] [PubMed]
- Suzuki Y, Okamoto T, Fujishita T, et al. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer 2016;101:92-7. [Crossref] [PubMed]
- Tsukioka T, Nishiyama N, Izumi N, et al. Sarcopenia is a novel poor prognostic factor in male patients with pathological Stage I non-small cell lung cancer. Jpn J Clin Oncol 2017;47:363-8. [Crossref] [PubMed]
- Nemec U, Heidinger B, Sokas C, et al. Diagnosing Sarcopenia on Thoracic Computed Tomography: Quantitative Assessment of Skeletal Muscle Mass in Patients Undergoing Transcatheter Aortic Valve Replacement. Acad Radiol 2017;24:1154-61. [Crossref] [PubMed]
- McDonald ML, Diaz AA, Ross JC, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc 2014;11:326-34. [Crossref] [PubMed]
- Tanimura K, Sato S, Fuseya Y, et al. Quantitative Assessment of Erector Spinae Muscles in Patients with Chronic Obstructive Pulmonary Disease. Novel Chest Computed Tomography-derived Index for Prognosis. Ann Am Thorac Soc 2016;13:334-41. [Crossref] [PubMed]
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755-63. [Crossref] [PubMed]
- Ma L, Xiang J. Clinical outcomes of video-assisted thoracic surgery and stereotactic body radiation therapy for early-stage non-small cell lung cancer: A meta-analysis. Thorac Cancer 2016;7:442-51. [Crossref] [PubMed]
- Rosen JE, Salazar MC, Wang Z, et al. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44-54.e9. [Crossref] [PubMed]
- Kudou K, Saeki H, Nakashima Y, et al. Prognostic Significance of Sarcopenia in Patients with Esophagogastric Junction Cancer or Upper Gastric Cancer. Ann Surg Oncol 2017;24:1804-10. [Crossref] [PubMed]
- Hervochon R, Bobbio A, Guinet C, et al. Body Mass Index and Total Psoas Area Affect Outcomes in Patients Undergoing Pneumonectomy for Cancer. Ann Thorac Surg 2017;103:287-95. [Crossref] [PubMed]
- Ikezoe T, Mori N, Nakamura M, et al. Effects of age and inactivity due to prolonged bed rest on atrophy of trunk muscles. Eur J Appl Physiol 2012;112:43-8. [Crossref] [PubMed]


