Disease-free survival improved by use of adjuvant EGFR tyrosine kinase inhibitors in resectable non-small cell lung cancer: an updated meta-analysis
Introduction
Lung cancer, with its high incidence and mortality, remains one of the leading causes of cancer-related death around the world. Although surgery is the main method to cure this disease, 30–70% of patients who have had en bloc resection may still relapse, and even develop distant metastasis (1). Therefore, the major current hypothesis holds that micro-metastasis occurs in the early phase of non-small cell lung cancer (NSCLC). Adjuvant chemotherapy can significantly increase overall survival (OS) rate by a scale of 4% to 5% among patients with high-risk stage IB and stage II–IIIA NSCLC (2,3), leading to the broad recommendation of adjuvant chemotherapy for those patients.
For advanced NSCLC patients, especially those harboring epidermal growth factor receptor (EGFR) activating mutations, EGFR tyrosine kinase inhibitors (TKIs) have been recommended as the standard first-line treatment, revealing better disease-free survival (DFS) and OS when compared with cisplatin-based chemotherapy following complete resection (4-6). Investigators from Memorial Sloan Kettering Cancer Center performed a multicenter single-arm SELECT trial (7) demonstrated the feasibility of adjuvant erlotinib in NSCLC patients with EGFR mutations, and found that EGFR-TKIs could improve survival in EGFR-mutant patients after surgery compared to historical controls (8). However, previous trials, BR19 (9) and RADIANT (10), without the inclusion of EGFR mutation status in patient selection criteria, yielded negative outcomes. In our previous meta-analysis, we reported that adjuvant EGFR-TKI treatment could enhance DFS in patients with EGFR-mutant NSCLC receiving complete resection (11).
This field has seen rapid advancement during the past years, and accumulating data have emerged. Among them, the phrase III ADJUVANT trial attracts the greatest attention. The recently reported results demonstrated that compared with cisplatin-based chemotherapy, adjuvant gefitinib treatment can significantly improve DFS, diminish toxic effects and enhance the quality of life in the included EGFR-mutant patients (12). A systematic review and meta-analysis were performed to evaluate whether the results of recently published or updated trials have changed the conclusions of our previous analysis.
Methods
Study eligibility and selection
All the studies from our previous meta-analysis was revaluated (11). The PubMed database was sought to identify potentially eligible studies published since September 2015, comparing the long-term outcomes of EGFR-TKI treatment with no EGFR-TKI treatment in adjuvant setting for patients with early-stage NSCLC after complete resection. The following search strategy was applied: (adjuvant OR postoperative) AND (epidermal growth factor receptor tyrosine kinase inhibitor OR EGFR-TKI OR Gefitinib OR Erlotinib OR icotinib) AND (Non-Small Cell Lung Cancer OR NSCLC OR Lung Neoplasms [MeSH] OR Adenocarcinoma of Lung). No language limitation was used.
Data extraction
All the corresponding data including the trial name, publication year, EGFR mutation rate, median duration of treatment, clinicopathological characteristics, treatment and outcomes were extracted from each selected study. The work of study selection and data extraction was carried out by two independent investigators (C Gu and Y Yuan), and a consensus was reached after discussion with a third author (Q Huang) for discrepancy.
Statistical analysis
Using the standard software (Stata 15.0, StataCorp LLC, College Station, Texas, USA), the meta-analysis was performed in line with recommendations from the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement.
The primary end point of the present study was DFS. DFS, which could avoid the influence of post-recurrence treatment, is being increasingly used as the primary end point in early-stage NSCLC randomized trials. Hazard ratios (HRs) with 95% confidence intervals (CIs) were used to express results related to survival data. For studies which did not report the HRs and their variances, methods by Parmar et al. and Tierney et al. were used to calculate the HRs and 95% CIs (13,14). Crude survival curves were estimated for the experimental group and the control group, to calculate the absolute DFS benefit at 3 years (15).
Heterogeneity was defined as significant difference when a χ2 P<0.1 or an I2>50%, and in this case the random-effects model was applied, or else the fixed-effects model was utilized (16). Besides, potential sources of heterogeneity were also detected among subgroup and meta-regression analysis. A P value <0.05 was considered statistically significant.
Results
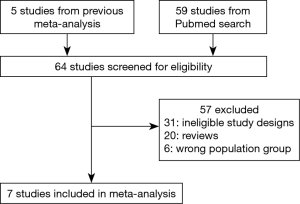
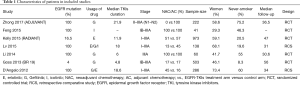
A total of 2,223 patients in seven studies were identified, including 1,151 patients in the EGFR-TKI treatment cohort and 1,072 patients in the control cohort. The selection process for eligible studies was shown in Figure 1. Studies with single-arm design were excluded. From Table 1, two retrospective studies were included, and the trials performed by Goss et al. (9) (BR19) and Kelly et al. (10) (RADIANT) applied EGFR-TKIs treatment without selecting positive EGFR-mutant patients, whose mutation rates were 4% and 16.5%, respectively. Moreover, the study BR19 was finished earlier (median, 4.8 months) than the original planned duration (2 years), therefore the participants enrolled was only approximately one-half as planned. The median TKIs treatment duration of ADJUVANT trial (median, 21.9 months) was the longest in ever known trials. Characteristics of patients enrolled were shown in Table 1.
Full table
Effects of EGFR-TKIs treatment on DFS in all patients
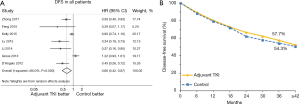
Compared with those without adjuvant EGFR-TKIs treatment, patients receiving adjuvant EGFR-TKIs gained a better DFS (HR, 0.60; 95% CI, 0.42–0.87) based on random-effect model (Figure 2A). Meanwhile, patients with EGFR-TKIs achieved an absolute benefit of 3.4% DFS at 36 months (Figure 2B).
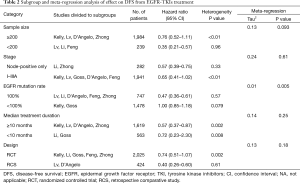
To explore the resource of heterogeneity, subgroup and meta-regression analysis of effect on DFS from EGFR-TKIs treatment were further conducted. The results were summarized in Table 2. Subgroup analysis showed that the effect of EGFR-TKIs upon DFS differed depending on whether the enrolled participants all harbored positive EGFR mutations or not, The difference was further confirmed by meta-regression analysis (P=0.005, Table 2). None of other potential contributors of heterogeneity was confirmed by meta-regression analysis.
Full table
Subgroup with EGFR mutations
Based on the outcomes of subgroup analysis, the effects of EGFR-TKIs in positive EGFR-mutant patients were further explored and the data of subgroup with EGFR mutations in BR19 and RADIANT trials were extracted. Pooled survival estimates revealed that the effect of adjuvant EGFR-TKIs was significantly better upon DFS (HR, 0.51; 95% CI, 0.39–0.65; Figure 3A) according to participants (386 in the EGFR-TKIs group, 537 in control group) harboring EGFR mutations of the seven studies. The results yielded a 7.1% absolute improvement in prognosis at 36 months (Figure 3B). No heterogeneity was found in the analysis (I2=11.7%, P=0.340) (Figure 3A).

Compliance and AEs
In the set of adjuvant EGFR-TKIs, 97.7% of patients received at least one dose of EGFR-TKI treatment. The BR19 trial was halted at the third year, and to some extent, the early shut of BR19 trial lower the rate indeed. The main reasons for unexpected treatment discontinuation were toxicity (17.4%). Eight percent of the patients in adjuvant EGFR-TKIs group refused the treatment after randomization.
Grade 3 or higher AEs were recorded in Table 3. Of all the patients treated with TKIs, the incidence rate of overall grade 3 or higher AEs was 38.9% (95% CI, 35.9–41.9%). Furthermore, the most common AEs were rash/acne (16.0%; 95% CI, 13.7–18.2%), diarrhea (5.8%; 95% CI, 4.3–7.2%) and dyspnea (4.4%; 95% CI, 3.0–5.8%). No grade 3 or greater interstitial lung disease was reported in trials. Three drug-related deaths occurred in EGFR-TKI group and all the three patients were from the BR19 trial.

Full table
Discussion
Improved DFS and reduced risk of distant metastasis of adjuvant EGFR-TKIs treatment for completely resected, EGFR-mutant NSCLC patients were shown in our previous meta-analysis (11). The present updated analysis included another two trials which were recently published, with 25.8% more patients than the previous analysis. Particularly, the patients of these two additionally trials were all EGFR-mutation positive, with an increase of 39.8% in the sample size of EGFR-mutant subgroup. The updated analysis revealed a reduction of 49% in the risk disease recurrence of adjuvant EGFR-TKIs treatment for EGFR-mutant patients, strengthening the current evidence of a survival benefit of EGFR-TKIs treatment in the adjuvant setting.
Molecular-targeted therapies have been successfully applied for adjuvant treatment in some types of neoplasms, such as imatinib for gastrointestinal stromal tumors (17,18), due to the excellent efficacy. Among completely resected NSCLC patients, adjuvant TKIs is supposed to work by obliterating the micro-metastases or residual tumor cells deriving from the primary neoplasm cells, which may share similar genotype and molecular. Therefore, TKIs could reduce disease relapse and improve OS theoretically. However, intra-tumor heterogeneity poses a real challenge to adjuvant molecular-targeted treatment, which is known as tumors comprise populations of cells with definite molecular and phenotypic features (19). In lung cancer, the evidence of branched evolution has accumulated, with the advent of driver mutations before and after subclonal diversification (20). When wild-type of the targeted genes appeared, molecular-targeted drugs would be unable to take effects to these tumor sub-clones.
As for the recently published ADJUVANT trial, the study population (stage II–IIIA NSCLC) was different from those (stage IB-IIIA) in previous trials (the BR19 and RADIANT). Whether stage IB NSCLC could benefit from adjuvant treatment existing controversies (21). Thus the ADJUVANT trial excluded stage IB patients and only included NSCLC patients with N1 or N2 disease, which may benefit from long-term adjuvant therapies (22). As previously reported, median OS for patients with N0, N1 and N2 stage lung cancer was 37, 21 and 9 months, respectively (23). Andre et al. (22) found that the median and 3-year DFS for patients with N2 disease was 12.2 months and 27%, respectively. The design of only including node-positive patients is considered to be another key reason for the inspiriting results, in addition to the inclusion of EGFR-mutant patients. We also found hints from the subgroup analysis of ADJUVANT trial. The survival advantage of EGFR-TKIs existed in the N2 subgroup, but not in the N1 subgroup, which is basically consistent with the results of subgroups analysis of the present study. These indicated that patients with more advanced disease might have higher chance to benefit from EGFR-TKIs. A pooled analysis of individual participant data is in demand to further confirm whether the benefit of adjuvant EGFR-TKIs varies over the tumor stage.
The timing of EGFR-TKIs administration also differed among different randomized controlled trials. In the BR19 trial (9) and RADIANT trial (10), EGFR-TKIs were compared with placebo in the adjuvant group and were supposed to explore efficacy of EGFR-TKIs after adjuvant chemotherapy. Conversely, in the SWOG S0023 trial (24), EGFR-TKIs were applied first, followed by definitely chemoradiotherapy. The design of the ADJUVANT trial was to confirm that gefitinib, one of EGFR-TKIs, could be a substitute for chemotherapy in the adjuvant setting. This is the first published trial to “head-to-head” compare adjuvant EGFR TKIs and adjuvant chemotherapy, and has the potential to change the current clinical management for resectable NSCLC.
The limitations of the study are as follows. Firstly, two retrospective studies were enrolled in the analysis, and the timing of adjuvant therapies administration were different among studies. Secondly, selection bias may occur in the study as only 71% patients of the EGFR mutation status in the BR19 trial were identified successfully. Moreover, individual patient data were not analyzed in the study, therefore, it is impossible to further explore the impact of some corresponding factors, such as age, stage, which could help deciding the optimal TKIs duration. These factors should be taken into consideration when interpreting the results of the study.
In summary, adjuvant EGFR-TKI treatment offers significant benefit to DFS. The optimal TKIs treatment duration and regimen still need further investigation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. J Clin Oncol 2014;32:abstr 7514.
- D'Angelo SP, Janjigian YY, Ahye N, et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 2012;7:1815-22. [Crossref] [PubMed]
- Goss GD, O'Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2015;33:4007-14. [Crossref] [PubMed]
- Huang Q, Li J, Sun Y, et al. Efficacy of EGFR Tyrosine Kinase Inhibitors in the Adjuvant Treatment for Operable Non-small Cell Lung Cancer by a Meta-Analysis. Chest 2016;149:1384-92. [Crossref] [PubMed]
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815-34. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists' Collaborative Group. Lancet 1992;339:1-15. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32:1563-70. [Crossref] [PubMed]
- Zhang J, Fujimoto J, Wedge DC, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014;346:256-9. [Crossref] [PubMed]
- de Bruin EC, McGranahan N, Mitter R, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science 2014;346:251-6. [Crossref] [PubMed]
- Luo J, Huang Q, Wang R, et al. Prognostic and predictive value of the novel classification of lung adenocarcinoma in patients with stage IB. J Cancer Res Clin Oncol 2016;142:2031-40. [Crossref] [PubMed]
- Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 2000;18:2981-9. [Crossref] [PubMed]
- Mauguen A, Pignon JP, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients' data. Lancet Oncol 2013;14:619-26. [Crossref] [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [Crossref] [PubMed]