The quest for immunotherapy in atherosclerosis: CANTOS study, interleukin-1β and vascular inflammation
The mighty cholesterol
Despite the monumental efforts directed into studying and describing the pathways, factors, genetic predisposition, and target-specific pharmacotherapy to atherosclerosis, ischemic heart disease, thrombotic cerebrovascular disease and peripheral artery disease are still responsible for 50% of all the deaths occurring in the developed world. The quest for a clear pathophysiology into atherosclerosis began with von Rokitansky’s incrustation theory, which evolved into the crucial role of platelets and thrombogenesis during acute coronary syndromes (1). Next, came the irritation theory postulated by Virchow, which detailed the presence of leukocytes in atherosclerotic plaques, suggesting the presence of chronic inflammation and progressive vessel deformation (1). By 1904, the term atherosclerosis was coined by Felix Jacob Marchand and 9 years later Nikolai Anichkov published that cholesterol alone can induce the vascular changes associated with atherosclerosis (1).
The impeccable work performed by Anichkov and his team, paved the way to the current understanding and clinical logic used in current cardiovascular medicine (the lipid hypothesis), which for the longest period focused mainly in plasma lipids as sole culprits for atherogenesis. Genetic connection between cholesterol and heart disease came in 1939, when Müller described families with severe hypercholesterolemia and early onset cardiac disease and death (2). The recognition of hereditary hyperlipidemias and their characteristics, cemented the role on cholesterol in cardiovascular risk, along with the findings from the epidemiological mammoth, the Framingham Heart Study (3). At some point, the lipid hypothesis was “universally recognized as a law” [2002] (4), and as such, it dominated pharmacotherapy development in cardiovascular medicine.
Niacin, the first lipid lowering drug, has generated favorable results regarding coronary atherosclerosis regression and reduction of cardiovascular events. However, its secondary effects have made it difficult to adhere to a long treatment plan. Following overwhelming evidence from several outstanding intervention trials (5), it’s been proven that statins are effective in controlling plasma lipids, including increase of high density lipoprotein (HDL-c), reducing cardiovascular disease, death from all causes and major cardiovascular events. Moreover, JUPITER trial demonstrated that primary prevention with rosuvastatin was possible in subjects without previous history of cardiovascular disease and elevated high-sensitivity C-reactive protein (hs-CRP) (5). As for fibrates, the absence of significant reduction in all-cause mortality and cardiovascular mortality has diminished the impact these drugs have, regardless of their multiple pleiotropic effects. Alternate lipid-lowering drugs (5) have been developed, for mono- or combined therapy, such as ezetimibe, bile acid sequestrants, cholesterol ester transfer protein (CETP) inhibitors, and proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors.
For the medical community, the lipid hypothesis dominance has rendered two standpoints: high fat diets elevate blood cholesterol, and in turn, hypercholesterolemia leads to atherosclerosis. For the patients, these indicate the need for life style changes including diet and pharmacological intervention to achieve cholesterol targets. However, the hypothesis is not ironclad as once thought, and laws must change, even in medical science. Several caveats can be mentioned, including (6): (I) cholesterol is associated to disease but does mean it is the sole culprit in atherosclerosis development; (II) disease causation is more than just one molecule, and the expansion of the mechanism of disease have aided in our understanding of vascular wall damage, neoantigens, infection factors, chronic inflammation and systemic disease; (III) plant-based diets have proven to be effective in reducing coronary artery disease without pharmacological intervention (7); (IV) elevated HDL-c is not associated with reduction of cardiovascular events (8).
Jaws and the immune system
The concept of vascular lipid deposit-foam cell formation-plaque enrichment-coronary events has been extended to include: (I) microbiome composition controls lipid plasma values and is liked to carotid atherosclerosis (9); (II) Chlamydia pneumonia infection has associated with atherosclerosis progression and its outer membrane protein has been suggested as a target in future antiatherosclerosis vaccines (10); (III) compelling results point to atherosclerosis as an autoimmune/autoinflammatory disease, sharing many components with other classical diseases such as inflammasomopathies (11); (IV) statin’s pleiotropic effects on hs-CRP and other markers of inflammation have hinted at an underlying inflammation hypothesis driving atherogenesis, including the discovery of geranylgeranyl pyrophosphate in innate system activation and oxidative stress (12), which fit the irritation theory. These statements point at an immunometabolic facet of atherosclerosis that seems to go beyond the lipid hypothesis. In fact, it completes the mechanism of disease, broadens therapeutic targets and gives reasoning to atypical cases and paradoxical data from different ethnic groups.
Even before the evolution of jawed vertebrates and the adaptive immune system, existing hosts could defend themselves against pathogens due to discrimination of self from non-self. The primitive defense systems included basic phagocytic ability, pathogen recognition receptors including Toll-like, Nod-like and scavenger receptors) adapted to pathogen- and damage-associated molecular patterns, and a myriad of pathogen-killing mechanisms which comprise reactive oxygen/nitrogen species, C-reactive protein, among others. The adaptive system was established in bony and cartilaginous fish, evolving from two unprecedented whole-genome duplication events, giving rise to the major histocompatibility complex (MHC) locus as we currently know, allowing the ultimate achievement—immunological memory.
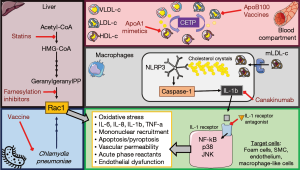
Given the complex control that the immune system exerts over homeostasis processes, it is no wonder that its role in atherogenesis involves every phase of the disease, and is still, partially understood. Briefly, endothelial dysfunction is the first step, followed by increased vascular permeability, monocyte recruitment and establishment/progression of localized chronic inflammation, leading to the different stages of atherogenesis. Several factors have been associated with endothelial dysfunction, including elevated and modified low density lipoproteins (mLDL-c) levels, smoking, hypertension, diabetes, infection such as Chlamydia and Cytomegalovirus (CMV) (13). Once LDL-c particles get trapped in the sub-endothelium, they are modified by oxidation, aggregation, glycation or are associated with immunocomplexes, serving as neoantigens/autoantigens. Modified LDL-c are taken up by macrophages, which will subsequently have phagolysosomal failure with cytosolic accumulation of crystalized cholesterol, establishing the foam cells (13). These fatty macrophages die via apoptosis, but many will succumb to secondary necrosis, acting as a positive loop reinforcing inflammation. The main immune response is TH1 dependent, with increased expression and secretion of interferon-γ, interleukin-6 (IL-6) and production of IgG2a antibodies against mLDL-c from Th1-dependent B2 cells (14).
To understand why IL1-β pathway has become one of the most promising new pharmacological targets, it is fundamental to understand the role of Nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome in foam cell death, and chronic vascular inflammation. The NLRP3 superstructure is expressed in several immunocytes, skin keratinocytes and epithelial monolayers of hollowed organs. This inflammasome requires priming for activation and ultimate production of IL-1β, IL-18 and IL-33 (15). Macrophages uptake mLDL-c via scavenger receptors, however the amount of engulfing is so that the phagolysosomal vesicle gets congested and destabilizes, causing lysosomal content leakage towards the cytosol. As a rule of thumb, cholesterol is an essential molecular for cell’s structure and viability, but its storage forms require molecular stabilization in the form of esters, via acyl-CoA: cholesterol acyltransferase. However, crystalized cholesterol is highly immunogenic and activates innate defense systems, such as the NLRP3 inflammasome. Cholesterol crystals are detected in the necrotic core of atherosclerotic plaque and in immunocyte-rich areas of the sub-endothelium, and are responsible for NLRP3 induction, IL-1β cleavage and secretion.
The IL-1β circuit requires three elements (Figure 1): IL-1β, IL-1 receptor type I and the IL-1 receptor antagonist. Once bound to its inflammatory receptor, IL-1β induces nuclear factor-κβ (NF-κβ), p38 mitogen-activated protein kinases (p38) and c-Jun activation and nuclear translocations, resulting in increased expression of IL-1 canonical proinflammatory mediators such as IL-6, IL-8, IL-1α and 1β, tumor necrosis factor-α (TNF-α), adhesion molecules, monocytes chemoattractant protein 1 (MCP-1), and hundreds more (15,16). In other words, IL-1β pathway drives a good portion vascular inflammation: foam cell formation, acute phase response reactants secretion, vascular permeability and mononuclear cell recruitment, and positive feedbacks that allow never-ending inflammation. In this context, immunotherapy targeting IL-1β pathway seems congruent with molecular biology of the disease; however, the pharmacological targets are three: the cytokine, its receptor or its natural antagonist.
Immunotherapy in atherosclerosis: a new perspective from the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS)
In this context, the work presented by Ridker et al., CANTOS (17), aims to determine if the inflammatory hypothesis is part of atherosclerosis pathogenesis, despite plasma lipid levels, and if anti-inflammatory measures could prevent secondary cardiovascular events in myocardial infarction (MI) survivors adhered to intense pharmacotherapy, but with persistent residual risk in the form of high hs-CRP levels (>2 mg/L). This randomized, double blind, placebo controlled study enrolled 10,061 patients (61.1±10.01 years old) and allocated them in four group, placebo and three different canakinumab doses (50, 150, and 300 mg), administered subcutaneous once every 3 months. The immunotherapy, canakinumab, is a human anti-IL-1β monoclonal IgGκ antibody developed by Novartis® as a neutralizing agent used in IL-1β-dependent diseases of autoinflammatory origin, such as inflammasomopathies, and autoimmune disorders like rheumatoid arthritis, systemic-onset juvenile idiopathic arthritis and gout arthritis. The primary efficacy end point was the first occurrence of nonfatal MI, any nonfatal stroke, or cardiovascular death in a time-to-event analysis. Secondary end points included the composite of the primary end points as well as hospitalization for unstable angina that led to urgent revascularization. The incidence of new-onset type 2 diabetes among patients with prediabetes at randomization was also considered as secondary endpoint.
Results showed that all three doses, 50, 150, and 300 mg, lowered hs-CRP levels (26%, 37% and 41% respectively) without any detectable impact of plasma lipid levels. However, the subjects receiving 150 mg every 3 months, achieved the pre-established endpoints and significant P thresholds, with 15% reduction of all primary end points compared to placebo groups (P=0.02075), and 17% reduction of secondary endpoint composites (P=0.00525). Interestingly, the 300-mg dose group obtained significant results but were not below the pre-established P thresholds. As predicted, canakinumab-treated groups presented serious side effects included increased risk of fatal infections/sepsis (P=0.02) and pseudomembranous colitis (P=0.03) when compared to the placebo group; other side effects include neutropenia (P=0.01) and thrombocytopenia (P=0.02). Patients who died from infection/sepsis were older and had diabetes; and six confirmed cases of tuberculosis occurred during the trial.
Albeit the overwhelming results, some caveats could be mentioned for this study. It could be argued that preventing secondary events might not be the best way to prove that the inflammatory hypothesis is the fundamental basis of atherogenesis. MI survivors have surpassed the point of no return in target organ damage and harbor several cofounding factors which could explain why hs-CRP remained high despite intensive pharmacotherapy. For example, aging is inexorably associated with the progressive loss of cardioprotective measures, with increased incidence in heart failure, loss of pacemaker cells, progressive arterial stiffness, among other variables. Incidence and prevalence of coronary and cerebral artery disease, atherosclerosis and hypertension is sharply increased at age 45 for men and age 55 for women (17); at age 70, the odds of having hypertension are 85%, for chronic heart failure 20% and for chronic cardiovascular disease are 50% (17). To use a secondary prevention stage to prove the inflammatory hypothesis in elderly patients might not be the best stage for it, especially when they are at higher risk for inherent immune senescence and higher risk of infection.
Interestingly, the CANTOS study did not report which MI subset did the treatment favored, i.e., ST-segment-elevation or non-ST-segment-elevation. Remarkably, the 150 mg canakinumab-treated group had a higher incidence of percutaneous coronary intervention (PCI) before randomization when compared to placebo. Noteworthy, is widely accepted that restenosis or neoatherosclerosis of the stented segments occur after PCI (18). Restenosis occurs due to remodeling and recoil of the vessel, while neoatherosclerosis is an accelerated form atherosclerosis that occurs drug-eluting stents. Even though these two pathological concepts are defined separately, neoatherosclerosis leads to vulnerable plaques, restenosis and post-stent complications. This information could suggest that patient who underwent PCI could have been disproportionally favored by receiving canakinumab, even perhaps the low dose group (18).
One interesting aspect of multiple factor aggregation in aging, is that they tend to group around overweight/obesity and/or a degree of adiposopathy (not only in the abdominal compartment, but more importantly in pericardial fat deposits) (19). Once the white adipose cell becomes sick, the organ becomes an “accidental” but important source of cytokines, including C-reactive protein, and IL-1β, and this secretome is also observed in sick epicardial fat (19) via nuclear factor-κB and c-Jun N-terminal kinases (JNKs) activation. The subjects in the CANTOS study were evenly overweight, with an interquartile range (IQR) that suggested obesity type I in some of them. It would be interesting to see how the effectivity of canakinumab might influence (or vice versa, becomes influenced) metabolic phenotypes, such as normal weight/overweight but metabolically obese and metabolically healthy obese subjects (20). As a matter of fact, IL-1 pathway blockade has been proposed to ameliorate insulin resistance and cardiometabolic risk for over 20 years, due to enhancement of adipose-derived low-grade inflammation. However, the CANTOS study offers no information regarding newly-developed diabetes, insulin levels or other markers of metabolic health, except plasma lipids.
Finally, the purpose of prevention lies in preventing the first major (even minor) cardiovascular event. However, to the cost of canakinumab in primary prevention might not be justifiable unless all the aspects of cardiovascular protection could be indeed “corrected” by IL-1 pathway blockade. canakinumab seems to be a probable candidate for secondary prevention in a specific group of patients, where benefits would certainly overcome the risks, including the high cost.
The future of atheroprotection
The results from the CANTOS trial are quite promising and reaffirm the need for new pharmaceutical developments in cardiovascular medicine in atheroma immunomodulation; note that we refer to immunomodulation to include strategies such as vaccines (10,11). Immunomodulation in atherosclerosis can have three basic targets: anti-inflammation, cholesterol synthesis and immunization against neoantigens (Figure 1). Briefly, anti-inflammatory therapies have included: blocking IL-1 receptor with anakinra in MI and left ventricle remodeling (21), administration of IL-1 receptor antagonist in neuroprotection [50], TNF-α blockers like etanercept in reduction of vascular inflammation (5), and IL-6 receptor blockers like tocilizumab (5). In regards to cholesterol synthesis inhibition and immunotherapy, PCSK9 inhibitors such as evolocumab, bococizumab and alirocumab (5). CETP inhibitors have been multiple yet they have failed to show efficacy in reducing mortality rates or cardiovascular events (5).
In regards anti-atherosclerosis vaccine development, several targets have been considered (10,11,22). ApoB-100-derived peptides, especially p210 prototype, have shown reduction of aortic plaques in murine models via activation of CD8+ T cells and Tregs (22). Several trials have been conducted using modified LDL as antigen, resulting in reduction of atherosclerosis burden (22). Another venue of investigation has been focusing in mononuclear cell recruitment and migration towards the sub-endothelium, such as the multi-epitope vaccine targeting CD99, CD81 and CD99L2 (23). Finally, DNA vaccine prototypes are also underway in animal models, including using CETP plasmid in a Hepatitis B virus core particle, resulting in reduction of aortic plaques in rabbits (24).
Conclusions
In conclusion, the CANTOS study demonstrates that inflammation is a fundamental trigger in vascular inflammation and is an ironclad pharmaceutical target for atheroprotection. The question for further investigations in to determine which anti-inflammatory method could be applicable to large populations and be used in primary and/or secondary prevention strategies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Capron L. Pathogenesis of atherosclerosis: an update on the three main theories. Ann Cardiol Angeiol (Paris) 1989;38:631-4. [PubMed]
- Müller C. Angina pectoris in hereditary xanthomatosis. Arch Intern Med 1939;64:675-700. [Crossref]
- Wilson PW, Garrison RJ, Castelli WP, et al. Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols. Am J Cardiol 1980;46:649-54. [Crossref] [PubMed]
- Thompson GR, Packard CJ, Stone NJ. Goals of statin therapy: three viewpoints. Curr Atheroscler Rep 2002;4:26-33. [Crossref] [PubMed]
- Bertrand MJ, Tardif JC. Inflammation and beyond: new directions and emerging drugs for treating atherosclerosis. Expert Opin Emerg Drugs 2017;22:1-26. [Crossref] [PubMed]
- DuBroff R, de Lorgeril M. Cholesterol confusion and statin controversy. World J Cardiol 2015;7:404-9. [Crossref] [PubMed]
- Esselstyn CB. A plant-based diet and coronary artery disease: a mandate for effective therapy. J Geriatr Cardiol 2017;14:317-20. [PubMed]
- Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012;380:572-80. [Crossref] [PubMed]
- Nakaya K, Ikewaki K. Microbiota and HDL metabolism. Curr Opin Lipidol 2018;29:18-23. [Crossref] [PubMed]
- Govea-Alonso DO, Beltrán-López J, Salazar-González JA, et al. Progress and future opportunities in the development of vaccines against atherosclerosis. Expert Rev Vaccines 2017;16:337-50. [Crossref] [PubMed]
- Matsuura E, Atzeni F, Sarzi-Puttini P, et al. Is atherosclerosis an autoimmune disease? BMC Med 2014;12:47. [Crossref] [PubMed]
- Vecchione C, Gentile MT, Aretini A, et al. A novel mechanism of action for statins against diabetes-induced oxidative stress. Diabetologia 2007;50:874-80. [Crossref] [PubMed]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med 1999;340:115-26. [Crossref] [PubMed]
- Zhou X, Paulsson G, Stemme S, et al. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest 1998;101:1717-25. [Crossref] [PubMed]
- Rojas J. Inflammasomes – Fighting the enemy from within. Avan Biomed 2012;1:18-29.
- Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal 2010;3:cm1. [PubMed]
- Ridker PM, MacFadyen JG, Everett BM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomized controlled trial. Lancet 2017. [Epub ahead of print]. [Crossref]
- Borovac JA, D’Amario D, Niccoli G. Neoatherosclerosis and late thrombosis after percutaneous coronary intervention: translational cardiology and comparative medicine from bench to bedside. Yale J Biol Med 2017;90:463-70. [PubMed]
- Salazar J, Luzardo E, Mejías JC, et al. Epicardial Fat: physiological, pathological and therapeutic implications. Cardiol Res Pract 2016;2016:1291537. [PubMed]
- Bermúdez V, Rojas J, Salazar J, et al. Sensitivity and Specificity Improvement in Abdominal Obesity Diagnosis Using Cluster Analysis during Waist Circumference Cut-Off Point Selection. J Diabetes Res 2015;2015:750265. [PubMed]
- Abbate A, Van Tassell BW, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction Am J Cardiol 2013;111:1394-400. [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. [Crossref] [PubMed]
- Chyu KY, Dimayuga PC, Shah PK. Vaccine against arteriosclerosis: an update. Ther Adv Vaccines 2017;5:39-47. [Crossref] [PubMed]
- Tourani M, Karkhah A, Najafi A. Development of an epitope-based vaccine inhibiting immune cells rolling and migration against atherosclerosis using in silico approaches. Comput Biol Chem 2017;70:156-63. [Crossref] [PubMed]
- Mao D, Kai G, Gaofu Q, et al. Intramuscular immunization with a DNA vaccine encoding a 26-amino acid CETP epitope displayed by HBc protein and containing CpG DNA inhibits atherosclerosis in a rabbit model of atherosclerosis. Vaccine 2006;24:4942-50. [Crossref] [PubMed]


