A technique of minimally invasive aortic valve replacement: an alternative to transcatheter aortic valve replacement (TAVR)
Introduction
Minimally invasive aortic valve surgery is increasingly being adopted by cardiac surgeons worldwide (1), in which aortic valve replacement (AVR) plays a central role. Since the first reported AVR occurred through a right thoracotomy in 1993 (2), a variety of minimally invasive techniques have been described, including right mini-thoracotomy (RT), mini-sternotomy (MS), transarterial and transcatheter AVR (TAVR). Among which, RT procedure has shown superior results in terms of mortality, morbidities and patient satisfaction when compared with traditional full sternotomy and MS approach (3,4). However, for patients with high surgical risk for advanced age and multiple comorbidities, RT approach will increase the cross-clamp and cardiopulmonary bypass (CPB) time for its limited exposure and working space. Furthermore, groin incision and rib division of this procedure also limits its application in these patients when compared with another minimally invasive method—TAVR. Nowadays, sutureless valves have evoked more and more interest, which has opened up a new field which is quite competitive with traditional AVR and TAVR. By quick implantation of the prosthesis which requires minimal suturing and tying, the cross-clamp and CPB time have been largely reduced, making this technique a competitive option for high risk patients. Here we present a surgical technique of minimally invasive AVR via RT using sutureless prosthesis without rib division and groin incision, providing a new surgical technique which not only can compare with the highly minimally invasive TAVR procedure, but also offer a more durable valve than TAVR.
Surgical technique
This study was deemed exempt by the Institutional Review Board at University of Florida. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A 60-year-old male with severe aortic stenosis (mean gradient: 51 mmHg; peak mean gradient: 80 mmHg; aortic valve area: 0.4 cm2) was identified as the candidate for this case. The patient elected to have a bioprosthetic valve placed due to his wish not to be on a long-term warfarin therapy. He presented New York Heart Association class III symptoms, and transthoracic echocardiography demonstrated severe impaired ejection fraction (30%). Coronary angiography result showed negative coronary lesion. Cardiac computed tomography imaging revealed severe calcification of aortic valve.
Surgical access was performed through an anterior RT through a 5-cm skin incision placed in the third intercostal space. Soft tissues were divided entering the right chest, and the right internal mammary artery and vein were sacrificed. For optimal exposure, a soft tissue retractor was used and no cartilage transection or rib dislocation was performed (Figure 1A). The pericardium was opened along the outer curvature of the aorta and extended towards the right atrium and inferior vena cava. Percutaneous femoral-femoral cardiopulmonary bypass was used in this case: using fluoroscopy, two guide wires were separately placed into the right common femoral artery and vein; a 21-French venous cannula was placed using the Seldinger technique and with the tip of cannula advancing into the superior vena cava under fluoroscopic guidance. Two separate Perclose devices were placed into the femoral artery and a 15-French arterial cannula was placed into the right external iliac artery over the guidewire. No groin incision was made (Figure 1B). A 5-mm stab incision in the right chest along the mid-axillary line provided access for a robotic Chitwood aortic cross-clamp.
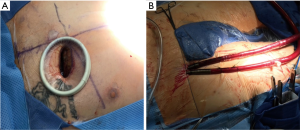
Cardioplegia was delivered anterograde via a Bengash needle and using del Nido solution. After adequate electromechanical cardiac arrest, the aortic valve was exposed through a transverse aortotomy. The native valve was excised and the annulus was thoroughly decalcified. Prosthesis was chosen after properly sizing the annulus, then the sutureless prosthesis (Perceval, medium size) was deployed at the target location following the manufacturer’s instructions. Mainly, 3/0 Prolene sutures are taken through the annulus at the nadir of each cusp, then passed through the three loops of inflow ring. The prosthesis was guided to correct position in annulus by sliding over these sutures. After the prosthesis being deployed, the delivery system and the guiding sutures were removed. A post dilatation balloon was inserted in the valve and dilated for 30 seconds at a pressure of 4 atmospheres to optimize the area of contact between the prosthesis and the aortic annulus. A suction catheter was used to vent the left ventricle by periodic insertion through the annulus. Carbon dioxide was used to flood the field throughout the case. A transesophageal echocardiogram (TEE) was routinely performed to verify correct prosthesis positioning. After the prosthesis replacement, the aortotomy was closed and the crossclamp was removed. The patient was weaned from CPB and all cannulas were removed. The arterial cannula site was closed with the two Perclose sutures. The pectoral muscle was readapted, and subcutaneous and intracutaneous sutures were applied for wound closure. A chest tube was placed through the site of the clamp in the right chest wall and temporary pacing wires were brought out through the chest tube incision.
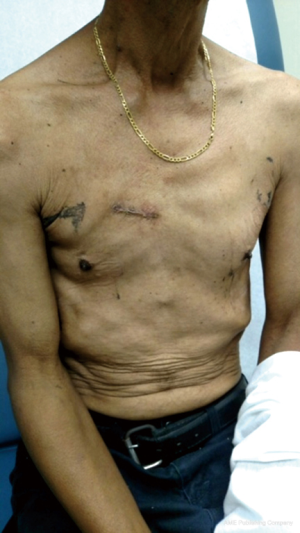
Cross-clamp and CPB times were 55 and 74 min respectively. The TEE showed a good valve coaptation with no paravalvular or central leak, and the post-operative mean gradient across the valve was only 3 mmHg. The patient needed no blood transfusion, and was discharged on the post-operative day 2 without any additional complication or pain complaining. At 2 weeks post-operation, the patient had no complaints at follow-up (Figure 2).
Discussion
Recent studies have demonstrated that minimally invasive AVR is a reliable technique as safe as standard sternotomy method. Moreover, it is associated with improvement in certain post-operative outcomes such as reduced length of stay (3), post-operative bleeding (5), mechanical intubation time (5), and wound dehiscence (6). Nowadays, TAVR has also become more and more accepted for its non-inferior results to surgical AVR and the recent FDA approval for high and intermediate risk patients (7). However, the main concern for TAVR still exists as paravalvular leak and valve long-term durability, and TAVR patients with moderate to severe aortic regurgitation had a significantly greater mortality at 2 years compared to its overall mortality (8). On the other hand, minimally invasive AVR has a very low rate of paravalvular leak and stands 20-year valve durability, which is potentially to be an alternative to TAVR in patients who require a minimally invasive approach and a durable valve.
Currently, the Miami Method is a common accepted minimally invasive method using RT approach, division of rib cartilage, rib retraction and trans-incisional aortic cross-clamping. Additional small incisions are made in the right lateral chest wall for retrograde cardioplegia, left ventricular venting and chest tube insertion; and femoral-femoral bypass is used via a longitudinal groin incision. However, Miami Method requires superior surgical technique, deep learning curves and relatively long operation time. On the basis of this method, we manage to present an easier and more minimally invasive approach. By using an aortic cross-clamp placed via a 5-mm chest tube incision, it allows for an unrestricted use of the thoracotomy which remains free of instruments. So, it can provide enough working space in a quite small operating field, and no rib division, rib retraction or related post-operative chest pain is necessary. Additionally, groin incision was avoided by femoral percutaneous technique, which is approximate to TAVR procedure which does not have a groin wounds. All these innovations lead to decreased trauma to the chest wall and groin, less post-operative pain and a faster recovery. Sutureless aortic valve is a new and promising tool for the treatment of aortic valve diseases. Deployment of a nitinol-stented sutureless bioprosthetic aortic valve allows for a further simplification to the surgical process. Besides shortening the cross-clamp/CPB time and avoiding complications due to prolonged extracorporeal perfusion, it showed great convenience in valve implantation in a very limited visual field and operating space. Because of the ease of implantation and quick insertion speed, it could increase applicability of minimal invasive aortic surgeries in the elderly with reduced cardiac reserve or with severe comorbidities. There have already been reported by several studies that the sutureless AVR represents a safe and effective treatment with excellent long-term follow-up results, especially suitable for patients with increased perioperative risk (9-11).
Therefore, by eliminating rib division and retraction, minimizing disruption in operating field, and avoiding a groin incision, this approach provides a minimally invasive and safe procedure in high-risk patients, causing less pain and faster recovery. By utilizing of a sutureless bioprosthetic valve, aortic cross-clamp and CPB time are also obviously reduced. To our knowledge it represents a quite minimally invasive AVR method via RT approach, which is quite reproducible in aortic valve surgeries. With the recent concerns about TAVR durability, we are offering patients an alternative minimally invasive approach with proven durability surgical valves.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Malaisrie SC, Barnhart GR, Farivar RS, et al. Current era minimally invasive aortic valve replacement: techniques and practice. J Thorac Cardiovasc Surg 2014;147:6-14. [Crossref] [PubMed]
- Rao PN, Kumar AS. Aortic valve replacement through right thoracotomy. Tex Heart Inst J 1993;20:307-8. [PubMed]
- Khoshbin E, Prayaga S, Kinsella J, et al. Mini-sternotomy for aortic valve replacement reduces the length of stay in the cardiac intensive care unit: meta-analysis of randomised controlled trials. BMJ Open 2011;1:e000266. [Crossref] [PubMed]
- Miceli A, Murzi M, Gilmanov D, et al. Minimally invasive aortic valve replacement using right minithoracotomy is associated with better outcomes than ministernotomy. J Thorac Cardiovasc Surg 2014;148:133-7. [Crossref] [PubMed]
- Murtuza B, Pepper JR, Stanbridge RD, et al. Minimal access aortic valve replacement: is it worth it? Ann Thorac Surg 2008;85:1121-31. [Crossref] [PubMed]
- Bonacchi M, Prifti E, Giunti G, et al. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg 2002;73:460-5; discussion 465-6. [Crossref] [PubMed]
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Breitenbach I, Wimmer-Greinecker G, Bockeria LA, et al. Sutureless aortic valve replacement with the Trilogy Aortic Valve System: multicenter experience. J Thorac Cardiovasc Surg 2010;140:878-84, 884.e1.
- Englberger L, Carrel TP, Doss M, et al. Clinical performance of a sutureless aortic bioprosthesis: five-year results of the 3f Enable long-term follow-up study. J Thorac Cardiovasc Surg 2014;148:1681-7. [Crossref] [PubMed]
- Sadowski J, Kapelak B, Pfitzner R, et al. Sutureless aortic valve bioprothesis '3F/ATS Enable'--4.5 years of a single-centre experience. Kardiol Pol 2009;67:956-63. [PubMed]


