Systemic air embolism after computed tomography-guided hook wire localization: two case reports and literature review
Introduction
Advances in computed tomography (CT) imaging technology and attempts to screen early lung cancer using low-dose CT facilitate the easy detection of small pulmonary nodules (1). These nodules are histopathologically diagnosed by less invasive procedures such as CT-guided transthoracic needle biopsy or endobronchial ultrasound-guided transbronchial needle biopsy. When histopathological diagnosis fails, then surgical approaches are needed. Most surgeons prefer video-assisted thoracoscopic surgery (VATS) to conventional open surgery due to lower invasiveness and comparable oncologic outcome. However, it is often difficult to identify small nodules using VATS, especially when the nodules are part-solid or non-superficial. CT-guided hook wire localization is commonly used for the accurate resection with low complication rate. Principal complications are pneumothorax, intrapulmonary hemorrhage, and hook wire dislodgement (2-9). Systemic air embolism (SAE) is a rare but potentially fatal complication, and only few cases have been reported worldwide. We report two recent cases of SAE at our hospital and review related literature.
Case presentation
Case 1
On November 2016, a 60-year-old woman visited our hospital for the treatment of a 9 mm-sized, part-solid nodule in the superior segment of the left lower lobe (LS6). The nodule was incidentally found during a routine medical check-up, although the patient had no relevant symptoms. The results of history taking, physical examination, laboratory tests, pulmonary function test, electrocardiography, and echocardiography revealed no abnormality. Because the nodule is centrally located in the lower region of LS6, we planned VATS segmentectomy of LS6 after hook wire localization, which was performed to estimate a sufficient resection margin from the nodule.
CT-guided hook wire localization was performed by an experienced interventional radiologist in the CT room just before transferring the patient to the operating room for surgery. The patient was placed in the prone position atop the CT table, and an initial CT image was obtained to determine the puncture site. After injecting a local anesthetic, a hook wire was delivered via 20 gauge needle (Accura™ Breast Localization Needle, Argon Medical Devices, Inc., Plano, USA). The CT scan was repeated to confirm the correct location of the wire and check for any possible complications.
The patient was stable and did not complain of any discomfort. There was no coughing or body movement during the procedure. However, the patient felt fatigue and generalized weakness after lying in the supine position, and the last CT image showed a small amount of air in the left atrium (Figure 1). While in the supine position, the patient was subjected to inhalation of 100% oxygen via a face mask. Electrocardiogram showed no unusual changes, and neurologic examination did not reveal any neurologic deficit, except for slight drowsiness.
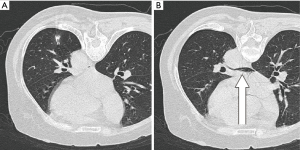
Symptoms disappeared completely after 10 min, but we monitored the patient for additional 20 min. We explained the current situation to the patient and her family, and the planned surgery was performed after obtaining their consent. VATS segmentectomy of LS6 with mediastinal lymph node dissection was uneventful. The patient did not show any neurologic symptoms or abnormal signs until discharge. The nodule was diagnosed as adenocarcinoma in situ.
Case 2
A 75-year-old woman visited our hospital on April 2017. Chest CT scans of a routine medical check-up revealed a 27 mm-sized tumor in the right upper lobe. Two other small nodules (measuring 2 and 3 mm in diameter) were found to be adjacent to each other in the right lower lobe. CT-guided needle biopsy confirmed the tumor in the right upper lobe as adenocarcinoma. Because preoperative evaluation did not reveal any contraindication, we scheduled VATS right upper lobectomy with mediastinal lymph node dissection followed by wedge resection of two small nodules in the right lower lobe after hook wire localization.
On the day of surgery, the localization was performed in a similar manner as that in the abovementioned case. The patient was in the prone position and nothing unexpected happened during the procedure. But the last CT scan showed a small amount of air in the left atrium (Figure 2). Nevertheless, the patient denied experiencing any discomfort, and we did not find any neurologic deficit or abnormal electrocardiographic change. After inhaling 100% oxygen via a face mask in the supine position for 20 min, the planned surgery was performed. The patient did not experience any complications after surgery, and was therefore discharged. Histopathologic study revealed stage IB (T2aN0M0) lung adenocarcinoma in the right upper lobe and cryptococcosis in the right lower lobe.
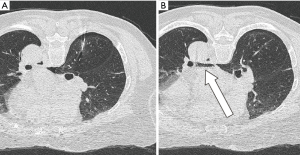
Discussion
Since its first introduction (10), the use of hook wire localization has gradually increased. It is especially useful when the nodule is relatively small, part-solid, or located far from the parietal pleura. Many studies have demonstrated the safety and effectiveness of hook wire localization. We analyzed the principal complications in these studies which included more than 50 cases and listed them chronologically in Table 1 (2-9). Major complications were pneumothorax, intrapulmonary hemorrhage, and wire dislodgement. In all studies, except in the study by Ciriaco et al. (4), pneumothorax was the most frequent complication (32.1–68.1%), but less than 4.6% patients needed treatments such as needle aspiration or tube thoracostomy. In cases of intrapulmonary hemorrhage, chest pain, or chest wall hematoma, symptoms were mostly mild and self-limited. In all studies, except one, the mortality rate was zero.
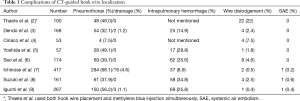
Full table
The only reported mortality was due to the acute myocardial infarction caused by SAE (11). SAE is a potentially fatal complication of hook wire localization; although much research has not been done regarding this, few case reports have been exist (7-9,11-15). Table 2 shows six cases found from the English literature search results.

Full table
In recent retrospective reports shown in Table 1, the estimated incidence rate of SAE was 0–0.62%. CT-guided lung biopsy is an analogous procedure in which a needle is inserted into lung parenchyma through the chest wall; this procedure gives rise to in SAE incidence rate of 0.12–0.49% according to recent reports (16-18). A noteworthy observation by Freund et al. (18) during their research involving 610 patients who underwent CT-guided needle biopsy was that although the reported incidence rate of SAE is 0.49% with a mortality rate of 0.16%, intravascular air on immediate post-procedural CT was prevalent in 3.8% of patients. This observation suggests that the incidence rate of asymptomatic or undiagnosed SAE in hook wire localization is higher.
Although there are no studies investigating mechanisms or risk factors of SAE, there have been some assumptions. Several researchers believe that air enters into the pulmonary vein while inserting a needle, or unfolding a hook wire, which likely occurs via communication between the peripheral airway and the pulmonary vein or communication between outside air and the pulmonary vein. Proposed risk factors are lesions located above the left atrium, deep lesions far from the visceral pleura, prone position, patient movement during procedure, repositioning after placement, and increased airway pressure due to cough, Valsalva maneuver, or positive pressure ventilation. Except for the case of one patient (13) who was under general anesthesia, all patients with SAE breathed spontaneously. Moreover, none of the cases were associated with unexpected movement, coughing, or poor patient cooperation. As shown in Table 2, majority of the nodules were presented in the lower lobe; this may be due to the fact that the lower lobe moves more than the upper lobe while breathing, which makes accurate localization difficult and increases the number of needle insertions.
SAE is a type of arterial embolism which causes ischemia. The main symptoms that appear in SAE are neurologic symptoms or cardiac symptoms. There is no report of the involvement of any other organs because small emboli in skeletal muscles or viscera are tolerable. Neurologic symptoms include seizure, dysarthria, limb paralysis, and visual field defect. Examples of cardiac symptoms are chest pain, bradycardia, hypotension, etc. Most of the patients showed complete recovery of symptoms within a few minutes to several hours by administration of a high fraction of oxygen. In a Japanese literature by Mizutani et al. (19), it has been reported that left hemiplegia did not improve even after several hours and was treated with hyperbaric oxygen therapy. Mortality due to coronary artery embolism (11) may occur due to the introduction of a significant amount of air into the vasculature, which is elucidated by an air-fluid level visible in the ascending aorta.
Early detection and prevention are important because available treatment is limited. The patient's condition should be frequently observed during the procedure, and abnormal symptoms, vital signs, and neurologic status of the patient should be evaluated after the procedure. To prevent unexpected body movement, sufficient amount of local anesthetics should be administered from the skin to the parietal pleura. After completion of the procedure, precautionary post-procedural CT should be taken to confirm the occurrence of any complications, including SAE. Suzuki et al. (8) provided patients with antitussives prior to the procedure and performed needle insertion with breath-holding under submaximal inspiration. If the inserted needle needs to be repositioned, then slow and careful movement is recommended. Ichinose et al. (7) changed their procedure strategy after they encountered SAE-related complication. They perform needle insertion after exhalation rather than inhalation, and seal the needle aperture and puncture lesion hermetically with jelly to prevent atmospheric air from entering through or around the needle.
Nonetheless, one cannot be sure that taking these precautionary measures will eliminate SAE risk because of the uncertainty regarding its mechanism. Therefore, we should be familiar with SAE management, and obtain informed consent about SAE risk after providing a detailed explanation. Once SAE is suspected, the patient should be administered on 100% oxygen under close monitoring. This can reduce the volume of embolus by aiding elimination of nitrogen. Hyperbaric oxygen therapy plays a similar role in treatment, and numerous case reports and review articles illustrate the potential mechanism and benefits of this therapy (20,21). The precise treatment position of a patient that will aid in enhancing air dispersion remains controversial. Right lateral position, Trendelenburg position, or flat supine position is recommended, but there is little supporting evidence (22-24). The buoyancy of air bubbles is not sufficient to counteract arterial blood flow; thus, such bubbles are propelled toward organs from the heart even when the patient is in the Trendelenburg position (25). If cerebral air embolism occurs, the Trendelenburg position has the potential to exacerbate the cerebral edema.
As shown in Table 2, the time from the onset of SAE to the surgery varies, and no one has commented on this issue. In three cases (9,12,13), after the symptoms completely recovered, no other complications occurred when the surgery was performed within two days after the procedure. On the other hand, in several other cases (7,8,14,15), after the surgery was postponed for approximately to 25 days. In all cases reviewed by us, there has been no explanation as to what action was taken with regard to the inserted hook wire. Because the possibility of recurrent embolism, bleeding, or pneumothorax due to the inserted hook wire while waiting for the surgery cannot be completely ruled out, we performed the surgery on the same day of insertion. Fortunately, no complications occurred in our cases. However, normalization of damaged vessels and organs requires time even if it is asymptomatic (24). Even if the inserted needle does not cause any additional complications, it is safer to closely monitor the stability of the patient for ≥1 days and to confirm the resolution of air emboli with subsequent CT and/or echocardiogram.
Agents such as microcoil, methylene blue, lipiodol, barium, and radioisotope can be used for localization. Electromagnetic navigation bronchoscopy can also assist in the placement of markers or dye materials to guide VATS. Use of ultrasound in the surgical field for detecting nodules has also been reported. However, the disadvantage of these techniques are lack of reproducibility and high associated costs. In a meta-analysis of comparing success and complication rates of hook wire, microcoil, and lipiodol localization methods, all three methods yielded highly successful targeting rates (26). SAE is a potentially fatal complication of hook wire localization, but it is rare and generally reversible with conservative treatment. With sufficient know-how and preventive measures, the most widely used hook wire localization technique need not be replaced due to the concern of SAE.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508-13. [Crossref] [PubMed]
- Thaete FL, Peterson MS, Plunkett MB, et al. Computed tomography-guided wire localization of pulmonary lesions before thoracoscopic resection: results in 101 cases. J Thorac Imaging 1999;14:90-8. [Crossref] [PubMed]
- Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Yoshida Y, Inoh S, Murakawa T, et al. Preoperative localization of small peripheral pulmonary nodules by percutaneous marking under computed tomography guidance. Interact Cardiovasc Thorac Surg 2011;13:25-8. [Crossref] [PubMed]
- Seo JM, Lee HY, Kim HK, et al. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg 2012;143:809-14. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. [Crossref] [PubMed]
- Suzuki K, Shimohira M, Hashizume T, et al. Usefulness of CT-guided hookwire marking before video-assisted thoracoscopic surgery for small pulmonary lesions. J Med Imaging Radiat Oncol 2014;58:657-62. [Crossref] [PubMed]
- Iguchi T, Hiraki T, Gobara H, et al. CT fluoroscopy-guided preoperative short hook wire placement for small pulmonary lesions: evaluation of safety and identification of risk factors for pneumothorax. Eur Radiol 2016;26:114-21. [Crossref] [PubMed]
- Mack MJ, Gordon MJ, Postma TW, et al. Percutaneous localization of pulmonary nodules for thoracoscopic lung resection. Ann Thorac Surg 1992;53:1123-4. [Crossref] [PubMed]
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Kamiyoshihara M, Sakata K, Ishikawa S, et al. Cerebral arterial air embolism following CT-guided lung needle marking. Report of a case. J Cardiovasc Surg (Torino) 2001;42:699-700. [PubMed]
- Horan TA, Pinheiro PM, Araújo LM, et al. Massive gas embolism during pulmonary nodule hook wire localization. Ann Thorac Surg 2002;73:1647-9. [Crossref] [PubMed]
- Sato K, Miyauchi K, Shikata F, et al. Arterial air embolism during percutaneous pulmonary marking under computed tomography guidance. Jpn J Thorac Cardiovasc Surg 2005;53:404-6. [Crossref] [PubMed]
- Iguchi T, Yoshioka T, Muro M, et al. Systemic air embolism during preoperative pulmonary marking with a short hook wire and suture system under CT fluoroscopy guidance. Jpn J Radiol 2009;27:385-8. [Crossref] [PubMed]
- Ibukuro K, Tanaka R, Takeguchi T, et al. Air embolism and needle track implantation complicating CT-guided percutaneous thoracic biopsy: Single-institution experience. AJR Am J Roentgenol 2009;193:W430-6. [Crossref] [PubMed]
- Kuo HL, Cheng L, Chung TJ. Systemic air embolism detected during percutaneous transthoracic needle biopsy: report of two cases and a proposal for a routine postprocedure computed tomography scan of the aorto-cardiac region. Clin Imaging 2010;34:53-6. [Crossref] [PubMed]
- Freund MC, Petersen J, Goder KC, et al. Systemic air embolism during percutaneous core needle biopsy of the lung: frequency and risk factors. BMC Pulm Med 2012;12:2. [Crossref] [PubMed]
- Mizutani E, Nakahara K, Miyanaga S, et al. Hyperbaric oxygen therapy for air embolism complicating computed tomography (CT)-guided needle marking of the lung. Kyobu Geka 2012;65:899-902. [PubMed]
- Muth CM, Shank ES. Gas embolism. N Engl J Med 2000;342:476-82. [Crossref] [PubMed]
- van Hulst RA, Klein J, Lachmann B. Gas embolism: pathophysiology and treatment. Clin Physiol Funct Imaging 2003;23:237-46. [Crossref] [PubMed]
- Mirski MA, Lele AV, Fitzsimmons L, et al. Diagnosis and treatment of vascular air embolism. Anesthesiology 2007;106:164-77. [Crossref] [PubMed]
- Kok HK, Leong S, Salati U, et al. Left atrial and systemic air embolism after lung biopsy: Importance of treatment positioning. J Vasc Interv Radiol 2013;24:1587-8. [Crossref] [PubMed]
- Jorens PG, Van Marck E, Snoeckx A, et al. Nonthrombotic pulmonary embolism. Eur Respir J 2009;34:452-74. [Crossref] [PubMed]
- Moon RE, de Lisle Dear G, Stolp BW. Treatment of decompression illness and latrogenic gas embolism. Respir Care Clin N Am 1999;5:93-135. [PubMed]
- Park CH, Han K, Hur J, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]


