Prognostic significance of histologic classification and tumor disappearance rate by computed tomography in lung cancer
Introduction
Recent advances in imaging technology and the widespread use of computed tomography (CT) screening have greatly increased the probability of detecting early small-sized lung adenocarcinomas with a ground-glass appearance (1). Adenocarcinoma with ground-glass opacity (GGO) generally has a good prognosis due to its minimally invasive nature (2,3). GGO feature corresponds closely to histopathologically lepidic growth patterns replacing the alveolar wall (2,3). In a new classification system recently proposed by the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS), adenocarcinomas with a lepidic growth pattern include adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and lepidic predominant invasive adenocarcinoma (LPA) (4). Many studies have shown that these pathologically less invasive subtypes are associated with better prognoses compared with other subtypes (5-9).
However, the percentage of lepidic growth pattern varies among tumors within the radiologically similar category of adenocarcinoma with GGO feature. A larger proportion of lepidic growth pattern correlates with better prognosis, but the presence of lepidic growth pattern per se does not guarantee that adenocarcinomas with GGO feature are always non- or minimally invasive. Nonetheless, few studies have thoroughly examined the histologic subtypes and the percentage of each subtype according to the new IASLC/ATS/ERS classification, especially focusing on adenocarcinoma with GGO feature.
Currently, the standard extent of resection for lung adenocarcinoma is lobectomy (10,11). However, multiple reports have suggested that small-sized peripheral lung adenocarcinoma can be treated by sublobar resection, yielding survival rates similar to those of lobectomy (12-15). However, when the extent of surgical resection is being decided either preoperatively or intraoperatively, it is difficult to accurately identify the predominant histologic subtypes because small biopsy samples do not provide sufficient material for comprehensive histological subtyping, considering the histologic heterogeneity of lung adenocarcinoma (5,16). To help surgeons decide the extent of pulmonary resection preoperatively, some researchers have attempted to predict pathologic invasiveness based on various radiologic parameters including the GGO ratio and the tumor disappearance rate (TDR) (17-20). Although several studies have investigated the relationship between radiologic parameters and pathologic features of lung adenocarcinoma based on the new IASLC/ATS/ERS classification system (21-25) few reports have focused on adenocarcinoma with GGO feature.
Therefore, we conducted a retrospective review of patients who underwent curative-intent surgery for lung adenocarcinoma with GGO morphology. The objectives of our study were (1) to investigate the prognostic value of the new IASLC/ATS/ERS classification system and (2) to assess the relationship between pathologic invasiveness and TDR.
Methods
Patients
Patients were included in the study if they had clinical T1-2N0 lung adenocarcinoma with GGO feature. GGO was defined as an area of a hazy increase in attenuation that did not obscure any underlying lung structures on CT scans. The consolidation component was defined as an area of increased opacification that completely obscured the underlying vascular markings. Pure GGO (no consolidation component) and mixed GGO (both a pure GGO and a consolidated region) were all included in the study. Patients were excluded if they had clinical T3, T4, N1, N2, N3, or M1 metastases; underwent resection for multiple tumors; received neoadjuvant treatment; or had prior pulmonary resection. After application of these criteria, between January 2000 and December 2009, a total of 202 patients were eligible for the study. The Institutional Review Board of Samsung Medical Center (2011-08-039-001) approved this study.
Staging workup and operation
Patients were staged according to the seventh edition of the American Joint Committee on Cancer staging manual (26) and the TNM classification manual from the International Union Against Cancer (27). Operative procedures included resection of the affected lung plus lymph node dissection of the ipsilateral hilum and the mediastinum. Lobectomy was chosen as the standard treatment, while segmentectomy or wedge resection was selectively performed when the tumor was considered to be resected with adequate resection margin. Patients were regularly evaluated by chest CT and/or PET/CT every 3 months for the first 2 years following surgery, and every 6 months thereafter.
TDR and histologic subtyping
The TDR (%) was defined as follows: [1-(maximum area of consolidation on the mediastinal window setting/maximum area of tumor on the lung window setting) x100]. Preoperative CT images were reviewed by a single radiologist blinded to pathologic results. All specimens were evaluated microscopically by a single pathologist blinded to clinical and radiologic data. Comprehensive histologic subtyping was performed according to the new IASLC/ATS/ERS classification system (4). As mentioned above, the study group was divided into 4 categories by histology; AIS, MIA, LPA, and non-lepidic predominant invasive adenocarcinoma (NLPA). AIS is defined as a solitary tumor ≤3 cm and pure lepidic pattern. MIA is also a solitary tumor ≤3 cm but predominantly lepidic. Moreover, an invasive component existed but should be ≤5 mm. If the tumor shows predominantly lepidic pattern, but could not be included in the AIS or MIA criteria, then it was defined as LPA. Other tumors which could not be included above criteria were defined as non-LPA. The percentage of each histologic component was recorded in 5% increments. The predominant pattern was defined as the pattern with the greatest percentage.
Statistical analysis
Student’s t tests or the Wilcoxon rank sum tests were used for continuous variables, whereas categorical variables were compared by the χ2 test or Fisher’s exact tests. One-way analysis of variance or the Kruskal-Wallis test was used to compare the continuous variables among the three groups. Overall survival (OS) was defined as the time from the date of surgical resection to death from any cause. Recurrence-free survival (RFS) was defined as the time from the date of surgical resection to recurrence or death. Survival curves were prepared using the Kaplan-Meier method and compared using the log-rank test. To determine prognostic factors, multivariate analysis was performed using the Cox proportional hazards model. All statistical tests were two-sided with a significance level set at 0.05 and were performed using Stata software version 10.0 (Stata, College Station, TX, USA).
Results
Patient data
Lobectomy was performed in 161 (80%) and sublobar resection in 41 (20%) patients. Patients who underwent sublobar resection had significantly larger areas of a lepidic growth pattern, less frequent NLPA, smaller tumor sizes, earlier pathologic T stages, less frequent pleural invasions and greater TDRs than those who underwent lobectomy (Table 1). The mean follow-up duration was 82.3 months (range, 2.2–180.8 months). During follow-up, 13 patients (6.4%) died and 12 (5.9%) developed recurrence. The 5- and 10-year OS rates were 96.9% and 89.6%, respectively. The 5- and 10-year RFS rates were 93.3% and 86.3%, respectively.
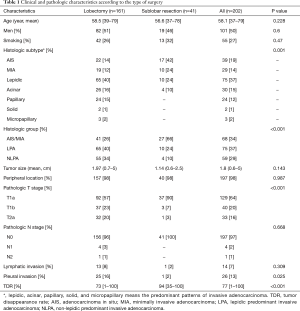
Full table
Comparative analysis according to histologic subtypes
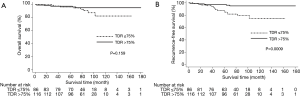
Thirty-nine patients (19%) had AIS, 29 (14%) had MIA, 75 (37%) had LPA, and 59 (29%) had NLPA (Table 2). Patients with NLPA had significantly smaller areas of a lepidic growth pattern, greater tumor sizes, more lymphatic and pleural invasion, and more advanced clinical and pathologic T stages (Table 2). There was no lymph node metastasis or lymphatic invasion in MIA but five patients had unexpected lymph node metastasis (4 pN1, 1 pN2), all of whom had invasive adenocarcinoma (2 LPA, 3 NLPA). Most recurrences occurred in patients with invasive adenocarcinoma (3 LPA, 7 NLPA), except for one with AIS and one with MIA. The 5-year OS and RFS rates were 99% and 95% in the AIS + MIA group, 96% and 95% in the LPA group, and 97% and 88% in the NLPA group, respectively (OS, P=0.069, Figure 1A; RFS, P=0.029, Figure 1B).
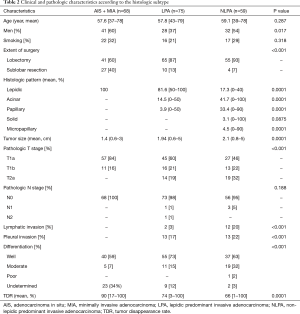
Full table

Comparative analysis according to TDR
The mean TDR was 90% in the AIS + MIA group, 74% in the LPA group, and 66% in the NLPA group (P<0.001, AIS + MIA vs. LPA, P=0.006, LPA vs. NLPA). ROC curve identified the optimal TDR cut-off value for predicting the pathologic invasiveness as 75% (AUC, 0.693; 95% CI, 0.63–0.76). The relationship between TDR and pathologic invasiveness was investigated using this cut-off value (Table 3). No patient with a tumor with a TDR >75% experienced recurrence. The 5-year OS and RFS rates were 97% and 97% in patients with tumors with a TDR >75% and 96% and 88% in a TDR ≤75%, respectively (OS, P=0.159, Figure 2A; RFS, P=0.0009, Figure 2B).
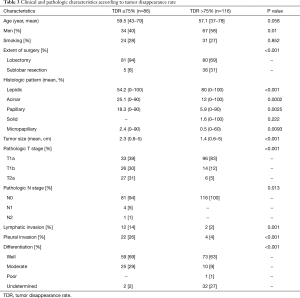
Full table
Prognostic factor analysis
Univariable analysis indicated that age, tumor size, and percentage lepidic growth pattern were significant prognostic factors for OS (Table 4). Moreover, age, tumor size, pathologic T and N stage, lymphatic invasion, percentage lepidic growth pattern, and TDR were significant prognostic factors for RFS (Table 4). The results of the multivariate analyses are summarized in Table 5. Tumor size and lepidic predominant growth pattern were identified as independent predictive factors associated with OS. Tumor size and lymphatic invasion were found to be independent predictive factors associated with RFS.
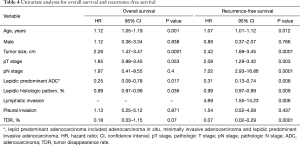
Full table
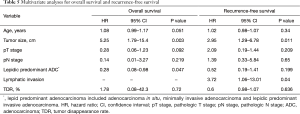
Full table
Discussion
In this study focusing on patients with GGO-type lung adenocarcinoma, more than 70% of the study cohort had lepidic predominant. This observation is in contrast to previous studies that included patients with lung adenocarcinoma irrespective of CT morphology. In previous reports, the frequencies of LPA ranged from 5.6% to 8.1% (5,7). Therefore, to the best of our knowledge, this study is the largest series of lepidic predominant adenocarcinomas for which the radiologic findings and pathologic features according to the IASLC/ATS/ERS classification system were comprehensively assessed.
We observed obvious prognostic differences between the histologic subtypes. Patients with AIS, MIA, and LPA had more favorable OS and significantly better RFS than those with NLPA. The differences in prognoses are possibly due to the pathologic invasiveness between them. Patients with AIS, MIA, and LPA had significantly smaller tumor sizes, earlier T stages, and lower incidences of lymphatic and pleural invasion than those with NLPA. No lymph node metastases were detected in any of the patients with AIS or MIA. As predicted, we also observed significant differences in the percentages of lepidic pattern between the histologic subtypes (AIS/MIA 100%, LPA 86.7%, NLPA 17.3%). Specifically, higher percentages of lepidic pattern were associated with lower risks of recurrence and better survival. This finding is consistent with previous reports (28,29).
The standard management for early-stage lung adenocarcinoma remains lobectomy (10,11). However, some suggested that small-sized peripheral lung adenocarcinoma can be treated by sublobar resection, yielding survival rates similar to those of lobectomy (12-15). In this study, among the 41 patients who underwent sublobar resection, more than 90% had lepidic predominant adenocarcinoma. Consequently, they had smaller tumor sizes, earlier T stages, lower incidences of pleural invasion, and lower percentages of lepidic growth pattern. These findings might be related to the tendency that we have conservatively selected the candidates for sublobar resection and thus suggest that patients with less aggressive pathologic features can be effectively treated with sublobar resection.
However, since accurate subtype can be known after surgery, radiologic parameters have been attempted to replace pathologic results (17-25). Takahashi et al. demonstrated that GGO ratio, TDR, and consolidation diameter were strong indicators of tumor invasiveness according to the new classification system (22). These findings were consistent with our study. We found that tumors with a TDR >75% were pathologically less invasive in terms of the predominant histologic subtype, tumor size, tumor invasiveness, and lymph node metastasis. Consequently, patients with tumors with a TDR >75% had excellent survival rates and no recurrence compared with those with tumors with a TDR ≤75%.
Our study has several limitations. First, since this was a retrospective study, selection bias was inevitable. As discussed above, patients who underwent sublobar resection had more favorable clinicopathologic characteristics than those who underwent lobectomy. This might have led to better survival outcomes in patients with tumors with a TDR >75% despite the fact that they received limited sublobar resection more frequently than others. Therefore, our data suggest that selected patients with AIS, MIA, and even LPA (or tumors with a TDR >75%) can be effectively treated with sublobar resection. Second, interobserver variability must be considered. This issue is especially relevant to the TDR calculations, since estimation of tumor size is subject to considerable variability given that the border of the GGO shadow might be difficult to define. To minimize potential variations in measurement, the radiologist in our study followed predefined window settings on the CT image for every calculation.
Conclusions
Here we comprehensively assessed radiologic parameters such as TDR and histologic subtype according to the new IASLC/ATS/ERS classification system in patients with GGO-type lung adenocarcinoma. We found that adenocarcinoma with a lepidic growth pattern was associated with less aggressive pathologic features and better survival outcomes. TDR was useful in predicting pathologic invasiveness and a lepidic growth pattern. Therefore, our data indicate that patients with tumors with ‘a less aggressive’ lepidic growth pattern and a high (>75%) TDR are good candidates for ‘the limited, but less invasive’ procedure of sublobar resection.
Acknowledgements
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2017R1C1B5015969).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board of Samsung Medical Center (2011-08-039-001) approved this study.
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [Crossref] [PubMed]
- Xu L, Tavora F, Battafarano R, et al. Adenocarcinomas with prominent lepidic spread: retrospective review applying new classification of the American Thoracic Society. Am J Surg Pathol 2012;36:273-82. [Crossref] [PubMed]
- Ito M, Miyata Y, Kushitani K, et al. Prediction for prognosis of resected pT1a-1bN0M0 adenocarcinoma based on tumor size and histological status: relationship of TNM and IASLC/ATS/ERS classifications. Lung Cancer 2014;85:270-5. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J 2009;33:426-35. [Crossref] [PubMed]
- Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. [Crossref] [PubMed]
- Koike T, Koike T, Yamato Y, et al. Prognostic predictors in non-small cell lung cancer patients undergoing intentional segmentectomy. Ann Thorac Surg 2012;93:1788-94. [Crossref] [PubMed]
- Carr SR, Schuchert MJ, Pennathur A, et al. Impact of tumor size on outcomes after anatomic lung resection for stage 1A non-small cell lung cancer based on the current staging system. J Thorac Cardiovasc Surg 2012;143:390-7. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Franks TJ, Galvin JR, Jett JR, et al. Expert opinion: role of percutaneous biopsy of part-solid nodules in the IASLC/ATS/ERS international multidisciplinary classification of lung adenocarcinoma. J Thorac Imaging 2011;26:189. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Correlation between computed tomographic findings, bronchioloalveolar carcinoma component, and biologic behavior of small-sized lung adenocarcinomas. J Thorac Cardiovasc Surg 2004;127:857-61. [Crossref] [PubMed]
- Nakayama H, Yamada K, Saito H, et al. Sublobar resection for patients with peripheral small adenocarcinomas of the lung: surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg 2007;84:1675-9. [Crossref] [PubMed]
- Hashizume T, Yamada K, Okamoto N, et al. Prognostic significance of thin-section CT scan findings in small-sized lung adenocarcinoma. Chest 2008;133:441-7. [Crossref] [PubMed]
- Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: A predictor of lymph node metastasis. J Thorac Cardiovasc Surg 2002;124:278-84. [Crossref] [PubMed]
- Honda T, Kondo T, Murakami S, et al. Radiographic and pathological analysis of small lung adenocarcinoma using the new IASLC classification. Clin Radiol 2013;68:e21-6. [Crossref] [PubMed]
- Takahashi M, Shigematsu Y, Ohta M, et al. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014;147:54-9. [Crossref] [PubMed]
- Liao JH, Amin VB, Kadoch MA, et al. Subsolid pulmonary nodules: CT-pathologic correlation using the 2011 IASLC/ATS/ERS classification. Clin Imaging 2015;39:344-51. [Crossref] [PubMed]
- Kudo Y, Matsubayashi J, Saji H, et al. Association between high-resolution computed tomography findings and the IASLC/ATS/ERS classification of small lung adenocarcinomas in Japanese patients. Lung Cancer 2015;90:47-54. [Crossref] [PubMed]
- Li Z, Ye B, Bao M, et al. Radiologic predictors for clinical stage IA lung adenocarcinoma with ground glass components: a multi-center study of long-term outcomes. PLoS One 2015;10:e0136616. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC. AJCC cancer staging manual.7th ed. New York: Springer, 2010:143-64.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of Malignant tumours, 7th Edition. Oxford: International Union Against Cancer and Wiley-Blackwell, 2009:269-72.
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Lee HY, Han J, Lee KS, et al. Lung adenocarcinoma as a solitary pulmonary nodule: prognostic determinants of CT, PET, and histopathologic findings. Lung Cancer 2009;66:379-85. [Crossref] [PubMed]


