Aberrant status and clinicopathologic characteristic associations of 11 target genes in 1,321 Chinese patients with lung adenocarcinoma
Introduction
Lung cancer is the most common malignancy and the leading cause of cancer-related deaths worldwide (1) whereas lung adenocarcinoma is the most frequent histological subtype of lung cancer (2). Oncogene aberrations have been extensively studied in the adenocarcinoma subtype (3), and show great molecular heterogeneity. With the approval of gefitinib, a first-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), in the early 2000s, the prognosis of the subgroup of patients with lung adenocarcinoma that harbor EGFR activating mutations has been dramatically improved. Subsequently, in addition to EGFR mutations, several target gene aberrations in lung adenocarcinoma have been discovered, including v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) (4), human epidermal growth factor receptor 2 (HER2) (5), B-raf proto-oncogene (BRAF) mutations (6), and anaplastic lymphoma kinase (ALK) translocations (7). The critical immunoregulator role of programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) has been extensively studied in lung cancers as well as many other cancer types. By using molecularly targeted agents for specific alterations, corresponding patients with lung adenocarcinoma have exhibited a significant and durable response (8).
The identifications of the prevalence of target gene alterations and their associations with clinicopathologic characteristics can make a significant difference in the selection of the therapeutic modality and the improvement of clinical outcome in lung adenocarcinoma patients. To date, though the target gene characterizations of lung adenocarcinoma have been reported in several separate studies; no large-scale research has been carried out systematically, either in China or worldwide, to analyze the status of target genes and its association with clinicopathologic characteristics. In the last year, we began to routinely examine the aberrant status of EGFR, KRAS, ALK, HER2, BRAF, Ret proto-oncogene (RET), ROS proto-oncogene 1 receptor tyrosine kinase (ROS1), phosphoinositide-3-kinase catalytic alpha polypeptide (PIK3CA), and neuroblastoma RAS viral (v-ras) oncogene homolog (NRAS), and the expressions of PD-1/PD-L1 in patients with primary lung adenocarcinoma who underwent surgical resection at our institution. Based on the clinical data that we have collected, we investigated the prevalence of the 11 target gene alterations and analyze their associations with clinicopathologic characteristics.
Methods
Ethics statement
This study was conducted with approval from the Ethics Committee of Zhongshan Hospital, Fudan University, Shanghai, China (Approval No. B2017-042). Written informed consent was obtained from all patients participating in this study at the time of hospitalization.
Patients
In this study, we screened all primary lung adenocarcinoma patients who underwent surgical resection of their tumors from April 2016 to March 2017, in the Department of Thoracic Surgery, Zhongshan Hospital, Fudan University. All the cases were clearly confirmed by pathologic evaluation. Of the 1,470 cases, both the expressions of PD-1/PD-L1 and the status of the nine target genes: EGFR, KRAS, HER2, ALK, BRAF, RET, ROS1, PIK3CA, and NRAS were detected in 657 patients. While the status of the nine target genes was analyzed in 590 different patients and the expressions of PD-1/PD-L1 in 74 different patients, respectively. A total of 1,321 patients were enrolled.
Target gene analysis
The status of the nine target genes and expressions of PD-1/PD-L1 were obtained from pathologists’ reports. In pathological examination, our institute routinely detects the status of the nine target genes in patients with lung adenocarcinoma using a detection kit for the mutation of human EGFR and another eight genes based on fluorescence real-time polymerase chain reaction (Amoy Diagnostics Co., Ltd., Xiamen, China) (Tables S1 and S2), including EGFR, KRAS, HER2, ALK, BRAF, RET, ROS1, PIK3CA and NRAS.
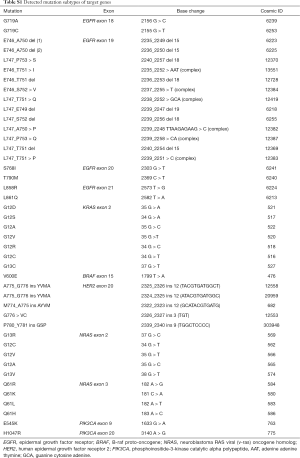
Full table
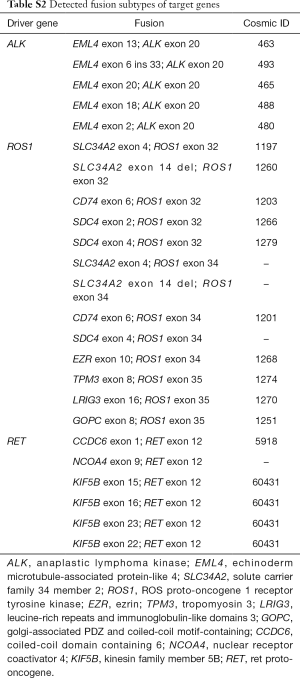
Full table
Immunohistochemical analysis
Immunohistochemical (IHC) staining of PD-L1 expression was performed on 4–6 µm thick formalin-fixed, paraffin-embedded tissue according to the manufacturer’s guidelines. Briefly, the primary antibodies specific for PD-L1 were applied to detect expression. Stained specimens were then viewed at 100× by the investigators. Gene expression was determined by assessing the percentage of marked cells as previously reported (9).
More than 5% of PD-1/PD-L1 positive cells in each specimen were considered positive because this percentage was reported to be related to the clinical response of anti-PD-1 therapy in a number of previous studies (10-13). Four antibodies were used to detect the expression of PD-L1, including 28–8, SP142, E1L3N, and BP6001. To simplify calculations, the ultimate number of PD-L1 positive cells were the average detected by more than one kind of antibody.
Clinicopathologic characteristics
Clinical data were obtained from patients’ electronic medical record database, including gender, age, smoking status, tumor location, tumour, node and metastasis (TNM) stage, histological subtype, and chest CT features. Histologic subtypes of lung adenocarcinoma were classified according to the new International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) multidisciplinary classification of lung adenocarcinoma (14). TNM stages were classified according to the revision of the 8th edition IASLC classification of TNM staging of lung cancer (15).
Statistical analysis
Correlations between target alterations and different subgroups stratified by sex, smoking status, tumor location, CT features, the classification of invasive adenocarcinomas, and the expressions of PD-1/PD-L1 were analyzed with chi-square and Fisher’s exact tests when appropriate. Independent two-sample t-tests were used to analyze the associations of age with the 11 target genes. Wilcoxon rank-sum tests were performed to analyze the associations of the 11 target genes with T stage, N stage, M stage, TNM stage, and histological subtypes. Associations of PD-1/PD-L1 positive tumor cells with positive stromal lymphocytes were analyzed with McNemar's test. Spearman’s rank correlation tests were used to analyze the correlations among the nine target gene aberrations and the expressions of PD-1/PD-L1. All tests were two-sided, and P values <0.05 were considered significant. All statistical analyses were performed using SPSS, version 24 (IBM, Armonk, NY, USA).
Results
Patient characteristics
A total of 1,321 patients with primary lung adenocarcinoma who had not received preoperative chemotherapy and targeted therapy were included in the present study. The demographics of all 1,321 patients with adenocarcinoma are listed in Table S3. Overall, 821 (62.1%) patients were women, 1,164 (88.1%) patients were never smokers, and the median age of all patients was 60 years (range, 24–85 years). Several patients had acinar predominant adenocarcinoma (n=748, 56.6%) and were at stage I (n=1,057, 80.0%). Additionally, 571 (43.2%) patients exhibited ground-glass opacity (GGO) or ground-glass nodule (GGN) in the CT images.
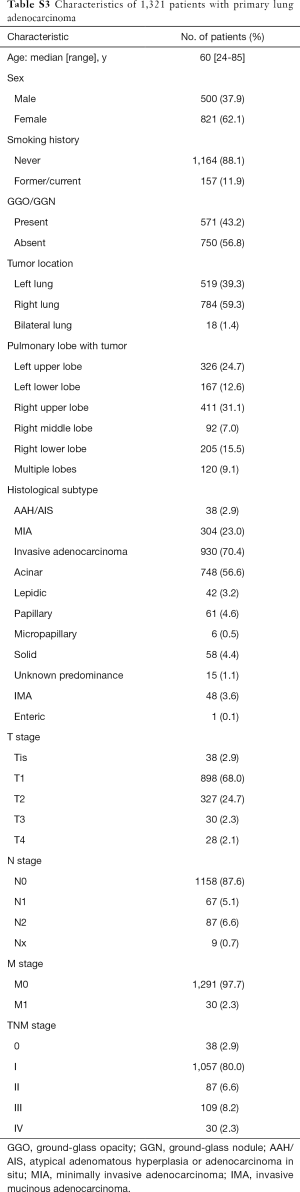
Full table
Prevalence of oncogene aberrations
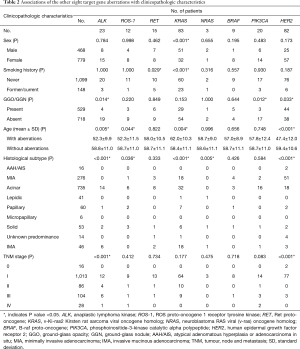
Of the 1,247 patients who had been examined to identify the mutations or translocations of the nine target genes, 952 (76.3%) patients harbored at least one gene aberration. Among these 1,247 patients, EGFR mutations were detected in 729 (58.5%) patients, KRAS mutations in 83 (6.7%) patients, HER2 mutations in 82 (6.6%) patients, while patients with alterations in the other six target genes were less than 2% (Tables 1 and 2). In addition, 24 (1.9%) patients harbored aberrations in two genes but none were found with aberrations in three or more genes.
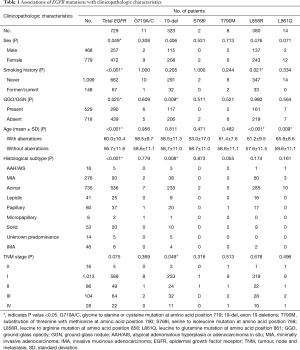
Full table
Full table
EGFR mutations status and associations with clinicopathologic characteristics
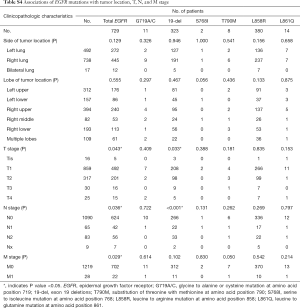
The most frequent subtype of EGFR mutation was a leucine to arginine mutation at amino acid position 858 (L858R) in exon 21, which occurred in 380 (30.5%) patients, followed by exon 19 deletions (19-del) in 323 patients (25.9%). The other four subtypes of EGFR mutations (G719A/C, S768I, L861Q) were all less than 1.2%, while eight (0.6%) patients harbored the substitution of threonine with methionine at amino acid position 790 (T790M) in exon 20 that relates to resistance to EGFR-TKIs. Additionally, there were 10 (0.8%) cases with two coexisting subtypes of EGFR mutations. No patient harbored three or more coexisting subtypes.
As shown in Table 1 and Table S4, EGFR mutations were significantly more frequent in women (P=0.049), patients who had never smoked (P<0.001), with the absence of GGO/GGN (P=0.025) and acinar predominant adenocarcinoma (P<0.001). Furthermore, patients with EGFR mutations were older than those without EGFR mutations (P<0.001). Stage T2, M1 were significantly associated with EGFR mutations (P=0.043, 0.029, respectively). No significant association was observed with tumor location.
Full table
EGFR L858R mutations were significantly associated with patients who had never smoked (P=0.021), while EGFR 19-del mutations were significantly associated with the absence of GGO/GGN (P=0.009). Furthermore, older age was significantly associated with EGFR L858R mutations (P<0.001) and EGFR L861Q mutations (P=0.009).
Associations of alterations in KRAS, HER2, ALK, ROS1, RET, BRAF, PIK3CA and NRAS with clinicopathologic characteristics
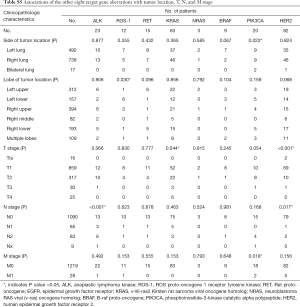
As Table 2 shows, KRAS mutations were significantly more frequent in men (P<0.001), and current or former smokers (P<0.001); while no significant correlation of gender was observed with other target gene alterations. In patients with GGO/GGN, there were significantly more frequent HER2 mutations (P=0.033), while less frequent ALK translocations (P=0.014) and PIK3CA mutations (P=0.012). In older patients, there were significantly more frequent KRAS mutations (P=0.004), while less frequent HER2 mutations (P<0.001), ALK translocations (P=0.005), and ROS1 translocations (P=0.044).
Invasive mucinous adenocarcinoma was significantly associated with KRAS mutations (P<0.001) and ALK translocations (P<0.001). Solid predominant adenocarcinoma was significantly associated with ROS1 translocations (P=0.036). No significant association was observed with pulmonary lobe harboring tumor, except ROS1 translocations (P=0.030). HER2 mutations and ALK translocations were significantly more frequent in stage 0 (P<0.001) and stage III (P<0.001), respectively (Tables 2,S5).
Full table
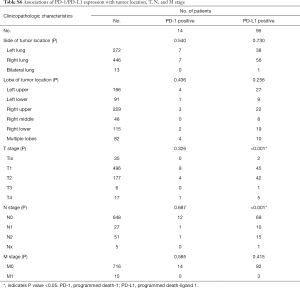
Associations of PD-1/PD-L1 expression with clinicopathologic characteristics
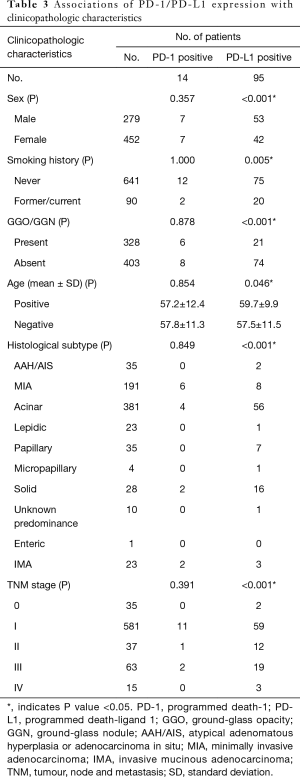
Of the 731 patients who had been examined to identify the expressions of PD-1/PD-L1, only 14 (1.9%) patients were PD-1 positive in tumor cells, yet 369 (50.5%) were PD-1 positive in stromal lymphocytes (P<0.001). Ninety-five (13.0%) patients were PD-L1 positive in tumor cells, yet 492 (67.3%) were PD-1 positive in stromal lymphocytes (P<0.001) (Table 3).
Full table
No significant associations of PD-1 positive tumors were observed with clinical features, which may be the result of the small number of patients with PD-1 positive tumors. PD-L1 positive tumors were more frequent in men (P<0.001), current or former smokers (P=0.005), patients without GGO/GGN (P<0.001), older patients (P=0.046), and those with solid predominant adenocarcinoma (P<0.001) (Tables 3,S6).
Full table
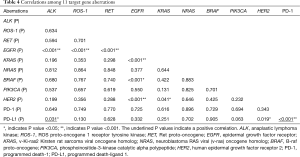
Correlations among the status of nine target genes and expression of PD-1/PD-L1
As Table 4 shows, of the 1,247 patients who had been examined to identify the status of the nine target genes, we observed that KRAS mutations, HER2 mutations, and ALK translocations were scarce in patients with EGFR mutations (P<0.001, respectively), particularly in patients with EGFR 19-del mutations and EGFR L858R mutations. Owing to the small number of ROS1 and RET translocations, PIK3CA, BRAF, and NRAS mutations, no obvious correlation was observed among them and other target aberrations.
Full table
PD-1 positive tumors positively correlated with PD-L1 positive tumors (P<0.001). PD-L1 positive tumors positively correlated with ALK translocations (P=0.031) and negatively correlated with HER2 mutations (P=0.019). There was no significant correlation of PD-L1 positive tumors with total EGFR mutation and its subtypes. (Tables 4,S7).

Full table
Discussion
In this study, we investigated the status of 11 common target genes in more than 1,000 lung adenocarcinoma patients. We found that over half of the patients harbored EGFR mutations, followed by KRAS mutations and HER2 mutations, which both occurred in approximately one in 15 patients. ALK, RET, and ROS1 translocations, PIK3CA, BRAF, and NRAS mutations were rare and all were identified in less than 2% of patients. PD-1 positive tumors were identified in 14 (1.9%) patients, and PD-L1 positive tumors in 95 (13.0%) patients. To the best of our knowledge, the present study is the first report elaborating such a comprehensive prevalence of nine target gene aberrations and the expressions of PD-1/PD-L1, as well as their associations with clinicopathologic features.
As reported previously, EGFR mutations, the most common target gene of lung adenocarcinoma, represented 75% of all the gene alterations and occurred more frequently in females, patients who had never smoked, the elderly, and acinar predominant adenocarcinoma (16-19). Interestingly, Hong et al. (20) found that EGFR mutations were significantly more frequent in tumors with GGO than in solid tumors, which was contrary to our findings. This may be the result of different sample sizes, areas where the participants live, and reagents used to detect the status of the EGFR gene. However, in our study, there were more women and fewer smokers (20). Additionally, the detected subtypes of EGFR mutations in our study were partly different (20). In the 729 EGFR-mutant patients, consistent with a previous study (21), EGFR L858R mutations and 19-del mutations were the most common subtypes, followed by EGFR L861Q mutations and EGFR G719A/C mutations. Chiu et al. (22) revealed that patients with EGFR G719X/L861Q/S768I mutations exhibited a significantly inferior tumor response rate and progression-free survival than patients with EGFR 19-del and L858R mutations after receiving gefitinib and erlotinib treatment, suggesting the limited efficacy of first-generation EGFR-TKIs in those patients. EGFR T790M mutations are the most common and well-characterized resistance mechanism of acquired resistance to gefitinib and erlotinib. Currently, osimertinib is the cornerstone for patients with EGFR-T790M mutations in second-line therapy (23). EGFR T790M mutations were identified in 1.1% of patients with mutant EGFR, indicating that this resistance may only occur in few patients with lung adenocarcinoma who have not received a preoperative targeted therapy.
KRAS mutations were identified in 83 (6.7%) patients, consistent with a previous report (24). Tanaka et al. and Xu et al. (24) demonstrated that KRAS mutations were significantly more frequent in males, current or former smokers, and the elderly, which was also found in the present study. No significant association between KRAS mutations and the presence of GGO/GGN was found, as previously reported (25). In agreement with a previous report, there was no significant association of HER2 mutations with gender and smoking status (26), whereas a significant association with younger patients was found (27).
Because of the small number of ALK translocations (n=23, 1.8%), PIK3CA mutations (n=20, 1.6%), BRAF mutations (n=9, 0.7%), NRAS mutations (n=3, 0.2%), ROS1 translocations (n=12, 1.0%), and RET translocations (n=15, 1.2%), agents targeting these target genes may be applied in the minority of patients with lung adenocarcinoma harboring these alterations.
Previous clinical trials found that the blockade of the PD-L1 and PD-1 interaction with specific antibodies had promising antitumor efficacy in patients with NSCLC (10,28). However, in our study, only a few patients were PD-1/PD-L1 positive. Azuma et al. (29) and Akbay et al. (30) found that EGFR mutations could up-regulate PD-L1 expression and that EGFR-TKIs could down-regulate PD-L1 expression. D’Incecco et al. (31) demonstrated that PD-1 positivity was significantly associated with current smoking status and with KRAS mutations, which was not observed in our study and needs to be further validated. Azuma et al. (29) found that expression of PD-L1 was significantly associated with females, patients who had never smoked, and EGFR mutations. Song et al. (32) also found that expression of PD-L1 was significantly associated with EGFR mutations, while no significant association was observed with other clinicopathologic parameters. Whereas, Jiang et al. (33) observed that PD-L1 expression in tumor cells was significantly higher in males, smokers, and patients with a higher histologic grade and that none of the EGFR, KRAS, MET, ALK and ROS1 gene abnormalities showed any statistical association with PD-L1 positivity. We also observed that PD-L1 positive tumors were significantly associated with males and current or former smokers, yet positively correlated with ALK translocations and negatively correlated with HER2 mutations. Various antibodies and the thresholds for PD-L1 positivity might lead to different results. Thus, further investigations to standardize the assay for PD-L1 expression are warranted.
There are some limitations in our study. Notably, the enrolled patients in the present study were mostly a Chinese Han population, so our results may not be applicable to people from other areas. Moreover, there was insufficient time to follow up the prognostic information. Therefore, we did not analyze the association of these target genes and corresponding targeted therapies with patients’ prognosis because the follow-up time was too short.
Conclusions
We demonstrated the prevalence of the 11 target genes and their comprehensive associations with clinicopathologic parameters in more than 1,000 Chinese lung adenocarcinoma patients. We hope our results will provide a reference for personalized medicine in patients with lung adenocarcinoma and improve their final prognosis.
Acknowledgments
We would like to thank the International Science Editing Co. for language editing.
Funding: This work was supported by the Training Programme for the Talents of Zhongshan Hospital, Fudan University (Grant No. 2015ZSYXGG03), and the National Natural Science Foundation of China (Grant Nos. 81370587, 81401875, 81672268, 31400713).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was conducted with approval from the Ethics Committee of Zhongshan Hospital, Fudan University, Shanghai, China (Approval No. B2017-042). Written informed consent was obtained from all patients participating in this study at the time of hospitalization.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Saito M, Shiraishi K, Kunitoh H, et al. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci 2016;107:713-20. [Crossref] [PubMed]
- Pasche B, Grant SC. Non-small cell lung cancer and precision medicine: a model for the incorporation of genomic features into clinical trial design. JAMA 2014;311:1975-6. [Crossref] [PubMed]
- Rodenhuis S, van de Wetering ML, Mooi WJ, et al. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med 1987;317:929-35. [Crossref] [PubMed]
- Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 2005;65:1642-6. [Crossref] [PubMed]
- Naoki K, Chen TH, Richards WG, et al. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res 2002;62:7001-3. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Hirsch FR, Suda K, Wiens J, et al. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016;388:1012-24. [Crossref] [PubMed]
- Zhan C, Yan L, Wang L, et al. Identification of immunohistochemical markers for distinguishing lung adenocarcinoma from squamous cell carcinoma. J Thorac Dis 2015;7:1398-405. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015;89:181-8. [Crossref] [PubMed]
- Tang Y, Fang W, Zhang Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 2015;6:14209-19. [Crossref] [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Li S, Choi YL, Gong Z, et al. Comprehensive characterization of oncogenic drivers in Asian lung adenocarcinoma. J Thorac Oncol 2016;11:2129-40. [Crossref] [PubMed]
- Wu YL, Zhong WZ, Li LY, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol 2007;2:430-9. [Crossref] [PubMed]
- Tanaka K, Hida T, Oya Y, et al. Unique prevalence of oncogenic genetic alterations in young patients with lung adenocarcinoma. Cancer 2017;123:1731-40. [Crossref] [PubMed]
- Dong YJ, Cai YR, Zhou LJ, et al. Association between the histological subtype of lung adenocarcinoma, EGFR/KRAS mutation status and the ALK rearrangement according to the novel IASLC/ATS/ERS classification. Oncol Lett 2016;11:2552-8. [Crossref] [PubMed]
- Hong SJ, Kim TJ, Choi YW, et al. Radiogenomic correlation in lung adenocarcinoma with epidermal growth factor receptor mutations: Imaging features and histological subtypes. Eur Radiol 2016;26:3660-8. [Crossref] [PubMed]
- VanderLaan PA, Rangachari D, Mockus SM, et al. Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: Correlation with clinical outcomes. Lung Cancer 2017;106:17-21. [Crossref] [PubMed]
- Chiu CH, Yang CT, Shih JY, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol 2015;10:793-9. [Crossref] [PubMed]
- Passaro A, Guerini-Rocco E, Pochesci A, et al. Targeting EGFR T790M mutation in NSCLC: From biology to evaluation and treatment. Pharmacol Res 2017;117:406-15. [Crossref] [PubMed]
- Xu J, He J, Yang H, et al. Somatic mutation analysis of EGFR, KRAS, BRAF and PIK3CA in 861 patients with non-small cell lung cancer. Cancer Biomark 2011-2012;10:63-9. [Crossref] [PubMed]
- Glynn C, Zakowski MF, Ginsberg MS. Are there imaging characteristics associated with epidermal growth factor receptor and KRAS mutations in patients with adenocarcinoma of the lung with bronchioloalveolar features? J Thorac Oncol 2010;5:344-8. [Crossref] [PubMed]
- Li X, Zhao C, Su C, et al. Epidemiological study of HER-2 mutations among EGFR wild-type lung adenocarcinoma patients in China. Bmc Cancer 2016;16:828. [Crossref] [PubMed]
- Suzuki M, Shiraishi K, Yoshida A, et al. HER2 gene mutations in non-small cell lung carcinomas: Concurrence with her2 gene amplification and her2 protein expression and phosphorylation. Lung Cancer 2015;87:14-22. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014;25:1935-40. [Crossref] [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 Pathway Contributes to Immune Escape in EGFR-Driven Lung Tumors. Cancer Discov 2013;3:1355-63. [Crossref] [PubMed]
- D'Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Brit J Cancer 2015;112:95-102. [Crossref] [PubMed]
- Song Z, Yu X, Cheng G, et al. Programmed death-ligand 1 expression associated with molecular characteristics in surgically resected lung adenocarcinoma. J Transl Med 2016;14:188. [Crossref] [PubMed]
- Jiang L, Su X, Zhang T, et al. PD-L1 expression and its relationship with oncogenic drivers in non-small cell lung cancer (NSCLC). Oncotarget 2017;8:26845-57. [PubMed]


