Fan-shaped ground-glass opacity (GGO) as a premonitory sign of pulmonary infarction: a case report
Introduction
Acute pulmonary embolism is one of the three major cardiovascular diseases along with cardiac and cerebral infarction and it has a high 30-day mortality rate in the order of ten percent (1,2). In general, typical symptoms such as shock, dyspnea and chest pain are caused by central pulmonary emboli at a higher frequency. However, patients with peripheral pulmonary emboli show minimal or no symptoms and early diagnosis is difficult (3). Pulmonary infarction is a necrosis of lung parenchyma caused by obstruction of the pulmonary artery and is seen in nearly 1/3 of pulmonary embolisms, especially segmental or more peripheral pulmonary emboli (4,5). In many cases, the onset of pulmonary infarction is unknown and local lesions in such cases tend to be surgically resected on clinical suspicion of lung cancer (6).
Radiological findings of pulmonary infarction have been well characterized mainly in established infarction (4,7). However, the early course CT appearance of patients who develop pulmonary infarction has not yet been fully elucidated due to the difficulty of an early diagnosis. We herein report a case of fan-shaped segmental ground-glass opacity (GGO) that was detected in the early phase of pulmonary infarction.
Case presentation
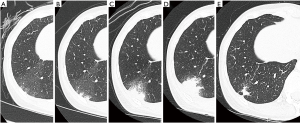
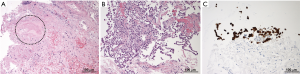
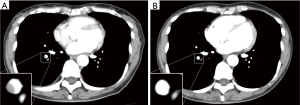
A 50-year-old female with a 1-year history of postmenopausal hormone replacement therapy (HRT) presented with dry cough for one week and visited a nearby hospital. The patient was diagnosed with pneumonia based on leukocytosis (10,400/µL) and high-resolution computed tomography (HRCT) findings of fan-shaped segmental GGO with parenchymal density in the right lower lobe (Figure 1). She was treated with clarithromycin followed by sitafloxacin. However, parenchymal density in the GGO gradually enlarged with trace amounts of bloody sputum and intermittent mild back pain over a period of 4 weeks (Figure 1). The patient was then referred to our hospital on clinical suspicion of bronchioloalveolar cell carcinoma. On admission, the patient presented with fatigue and a low grade fever; the significant laboratory findings were CRP of 2.15 mg/dL and D-dimer of 1.2 µg/mL. We discontinued the HRT since many large clinical studies have shown that HRT promotes venous thromboembolism [(VTE): deep venous thrombosis and/or pulmonary embolism] by affecting hemostatic balance and inflammation (8). The patient did not have any other risk factors for VTE such as obesity, active cancer, trauma or hospitalization for surgery or acute illness. Enhanced CT and whole-leg ultrasonography could not detect any VTE. However, a transbronchial biopsy of the lesion revealed necrosis of the pulmonary tissue, including the small pulmonary artery (Figure 2A). Reactive proliferation of the calretinin-positive pleural mesothelium was also observed (Figure 2B,C). These pathological findings were compatible with pulmonary infarction (9,10). After the endoscopic examination, parenchymal density enlarged causing a triangular structure with a broad pleural base surrounded by narrow GGO. A linear strand at the apex of the lesion and a small pleural effusion were also observed (Figure 1). The radiological findings were all described in previous studies investigating typical CT appearance of established pulmonary infarction (4,7). Moreover, enhanced CT detected an intraluminal filling defect in the right lower lobe artery suggesting peripheral pulmonary emboli (Figure 3A), although whole-leg ultrasonography could not detect deep vein thrombosis. We speculated that previously undetected residual thrombosis upstream from the infarction had grown and anti-coagulant therapy with rivaroxaban was started (11,12). Three months later, the thrombotic burden had disappeared (Figure 3B) and parenchymal density was reduced to a small irregular shadow implying scar formation (Figure 1).
Discussion
CT findings, such as decreased parenchymal enhancement, broad pleural base, truncated apex, convex border, low-attenuation areas within peripheral consolidations (internal lucencies), linear stranding from the apex toward the hilum, and lower lobe predominance, have been reported as characteristic features of established pulmonary infarction (4,7). As for internal lucencies, these images are thought to indicate viable lung tissue coexisting side-by-side with infarcted lung or cavitation in the infarcted lung (4,7) . However, little attention has been paid to faint and relatively homogeneous GGO in the early phase. The redistribution of blood flow after obstruction of the central pulmonary artery can induce a mosaic pattern of perfusion in the unobstructed lung zones of patients with chronic pulmonary thromboembolism. The characteristic CT findings in such cases are sharply demarcated regions of increased and decreased attenuation (13). A similar phenomenon was reported in a pig model of acute pulmonary embolism (14). However, most fan-shaped segmental GGO observed in our case gradually progressed to the smaller, triangular shape of established pulmonary infarction. The time course of the CT evolution implied that GGO was a premonitory sign of pulmonary infarction in the early phase, but was not due to hyperperfusion. Ischemia of lung tissue leads to marked dilatation of blood vessels in the microcirculation system, accompanied by increased vascular permeability causing leakage of fluid and erythrocytes before tissue necrosis (9). These pathological changes were probably factors in the GGO in this case.
Although GGOs are usually observed accompanied by other interstitial or airspace patterns, various diseases lead to GGOs without other findings or as the predominant feature. Such diseases can be classified into four groups: opportunistic infections (e.g., pneumocystis pneumonia and cytomegalovirus pneumonia); interstitial diseases (e.g., hypersensitivity pneumonitis, nonspecific interstitial pneumonia and acute interstitial pneumonia); acute alveolar diseases (e.g., pulmonary edema and diffuse alveolar hemorrhage); and unusual miscellaneous disorders (e.g., pulmonary alveolar proteinosis, cryptogenic organizing pneumonia, eosinophilic pneumonia and bronchioloalveolar carcinoma) (15). However, these diseases, other than bronchioloalveolar carcinoma, rarely present a solitary lesion and, to the best of our knowledge, fan-shaped GGO has not been reported prior to this manuscript.
Conclusions
Our case was a peripheral pulmonary infarction, probably induced by HRT, and it suggested that fan-shaped GGO may be a premonitory sign of pulmonary infarction. The accumulation of similar cases is necessary to evaluate the specificity and sensitivity of fan-shaped GGO as a premonitory sign of pulmonary infarction in the early phase.
Acknowledgements
We would like to thank Shinya Hatono (Division of Radiology, National Hospital Organization Kochi Hospital) for his contribution.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Scherz N, Labarère J, Méan M, et al. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010;182:1178-83. [Crossref] [PubMed]
- Bach AG, Taute BM, Baasai N, et al. 30-day mortality in acute pulmonary embolism: prognostic value of clinical scores and anamnestic features. PLoS One 2016;11:e0148728. [Crossref] [PubMed]
- Yoo HH, Queluz TH, El Dib R. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst Rev 2016.CD010222. [PubMed]
- He H, Stein MW, Zalta B, et al. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006;21:1-7. [Crossref] [PubMed]
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982;72:599-606. [Crossref] [PubMed]
- George CJ, Tazelaar HD, Swensen SJ, et al. Clinicoradiological features of pulmonary infarctions mimicking lung cancer. Mayo Clin Proc 2004;79:895-8. [Crossref] [PubMed]
- Balakrishnan J, Meziane MA, Siegelman SS, et al. Pulmonary infarction: CT appearance with pathologic correlation. J Comput Assist Tomogr 1989;13:941-5. [Crossref] [PubMed]
- Eisenberger A, Westhoff C. Hormone replacement therapy and venous thromboembolism. J Steroid Biochem Mol Biol 2014;142:76-82. [Crossref] [PubMed]
- Wagenvoort CA. Pathology of pulmonary thromboembolism. Chest 1995;107:10s-7s. [Crossref] [PubMed]
- Cagle PT, Churg A. Differential diagnosis of benign and malignant mesothelial proliferations on pleural biopsies. Arch Pathol Lab Med 2005;129:1421-7. [PubMed]
- Quiroz R, Kucher N, Zou KH, et al. Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. Jama 2005;293:2012-7. [Crossref] [PubMed]
- EINSTEIN–PE Investigators, Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97. [Crossref] [PubMed]
- Castañer E, Gallardo X, Ballesteros E, et al. CT diagnosis of chronic pulmonary thromboembolism. Radiographics 2009;29:31-50; discussion 50-3. [Crossref] [PubMed]
- Thoma P, Rondelet B, Mélot C, et al. Acute pulmonary embolism: relationships between ground-glass opacification at thin-section CT and hemodynamics in pigs. Radiology 2009;250:721-9. [Crossref] [PubMed]
- Miller WT Jr, Shah RM. Isolated diffuse ground-glass opacity in thoracic CT: causes and clinical presentations. AJR Am J Roentgenol 2005;184:613-22. [Crossref] [PubMed]


