Validation of the T descriptor in the new 8th TNM classification for non-small cell lung cancer
Introduction
The accurate staging of lung cancer is an essential diagnostic step that influences the choice of neoadjuvant and adjuvant therapy and the intraoperative surgical strategy. The TNM system describing the anatomical extent of malignant tumors is one of the most widely adopted cancer staging systems, and is used by most medical facilities as their main method for cancer reporting.
The initial TNM staging system for lung cancer was first adopted by the American Joint Commission on Cancer (AJCC) in 1973 and by the Union for International Cancer Control (UICC) in 1974 (1). The current seventh edition of the TNM system for lung cancer was based on an international database of 81,495 patients collected between 1990 and 2000 by the International Association of the Study of Lung Cancer (IASLC) (2). The changes proposed by the IASLC were fully approved by both the UICC and the AJCC and are reflected in the current seventh edition of the TNM staging system. However, despite the vastness of the IASLC database and various external validations by independent groups (3,4), not all descriptors of the T component could be validated because many of the contributing databases had not been designed to analyze the TNM system. The resulting lack of detailed data necessitated the collection of new data.
The new eighth edition of the TNM system is the product of an extensive initiative by the IASLC, involving a database of 94,708 patients, although it has not yet been externally validated (5). Here, we describe the first study attempting to validate the revisions of the T descriptors in the forthcoming eighth edition of the TNM system for lung cancer. We reviewed the survival characteristics of patients and compared the predictive abilities of the newly proposed T descriptors for non-small cell lung cancer (NSCLC).
Methods
Patients
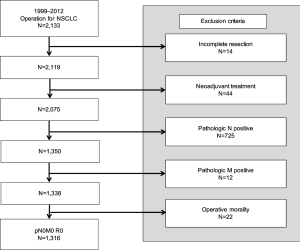
A total of 2,133 consecutive patients who underwent surgery for NSCLC between January 1999 and December 2012 at our institute were analyzed. Of these, patients for whom an incomplete resection (R1 or R2 resection) could be performed, who underwent preoperative chemoradiation therapy, who died within 1 month following surgery, or who had a tumor with histology other than NSCLC were excluded. Patients who had a positive nodal status or distant metastasis were also excluded. When the ground glass opacity (GGO) lesion, which had 5 mm or more solid component, was detected on computed tomography (CT) scan, we considered a biopsy or surgical resection. If GGO lesion was diagnosed as pre-invasive lesion such as atypical adenomatous hyperplasia (AAH) and adenocarcinoma in situ (AIS), we observed without further definitive surgery and excluded from this study. Consequently, 1,316 patients were included in the analysis of the pathologic T category (Figure 1). This study was reviewed and approved by the Institutional Review Board at Yonsei University Health System, Severance Hospital (No. 4-2017-0935).
Statistical analysis
Overall survival was defined as the interval between the date of surgery and either the date of death from any cause or the last clinical visit. The Kaplan-Meier method was used to estimate the overall survival in the different T categories. The significance of differences between the survival curves was calculated using the log-rank test.
We used Harrell’s concordance (C)-index and Heagerty’s integrated area under the curve (iAUC) analysis to assess the discriminatory abilities of the different TNM staging systems (6,7). The C-index has been widely used for assessing predictive values in survival analysis, and has a value between 0.5 (no discrimination) and 1.0 (perfect discrimination). The estimated iAUC compares the predictive accuracy for time to event data using the rank sum test method for dependent samples when the random censoring assumption holds.
All reported p values were 2-sided, and a value of P<0.05 was considered statistically significant. SPSS 20 for windows v20.0 (SPSS Inc., Chicago, IL, USA) and R package version 3.0.1 (http://www.R-project.org) were used for statistical analyses.
Results
An overview of the current and proposed T category definitions is given in Table 1. The study cohort of 1,316 patients included 500 (38.0%) female and 816 (62.0%) male patients, with a mean age of 63.1±0.3 years. Lobectomy was performed in 1,131 (85.9%) patients, and 1,249 (95.0%) patients were pathologically confirmed to have adenocarcinoma or squamous cell carcinoma. The patients’ baseline characteristics are summarized in Table 2, and the distribution of cases according to seventh and newly proposed eighth TNM staging system is given in Table 3. Under the eighth TNM staging system, 771 (58.6%) patients were assigned to a higher pathologic stage and 16 (1.2%) to a lower one.
The 5-year survival rate and the survival curves according to the T descriptors of the seventh and eighth editions are shown in Figure 2. In the seventh edition, the survival curves showed a stepwise deterioration as the pathologic stage increased except between T1b and T2a (P=0.874), and T3 and T4 (P=0.943). However, there were no significant survival differences between each stage based on the T stage criteria of the eighth edition, most notably between T1a and T1b (P=0.752), and T1c, T2a, and T2b (P=0.832).

Comparison of the TNM prognostic classification systems
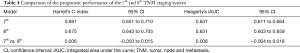
Statistical assessment of the prognostic performance of two TNM staging editions using the C-index revealed a value of 0.681 (95% CI, 0.651–0.710) for the seventh edition, and 0.675 (95% CI, 0.643–0.705) for the eighth edition. We found no significant improvement in prognostic accuracy between the seventh and eighth editions (Table 4).
Full table
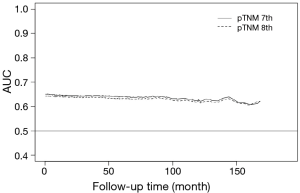
Moreover, we calculated the AUC after surgery for each follow-up period. A larger iAUC indicates a better average predictability of time to event. The iAUC values of two TNM staging editions varied between 0.59 and 0.64, and the eighth edition also showed no relevant difference in the iAUC value compared with the seventh edition (Figure 3). The newly proposed eighth edition of the TNM staging system thus did not predict survival more accurately than the seventh edition.
Discussion
In this study, we performed a retrospective analysis in order to assess whether the prognostic significance of the newly proposed T descriptors of the eighth TNM staging system in patients who underwent surgery for NSCLC was greater than those of the previous TNM staging systems. The T descriptor in the eighth edition showed narrower discrimination between sequential stage groups and did not have a better prognostic performance than the seventh edition. Based on these results, the newly proposed T descriptors in the forthcoming eighth edition of the TNM classification for NSCLC were not superior with respect to prognostic stratification amongst patients from our institution.
Although the current seventh TNM staging system for NSCLC adopted in 2010 was based on a database of more than 80,000 patients collected worldwide, not all descriptors could be validated. For the T category, only tumor size, additional tumor nodules, and pleural effusion could be analyzed reliably, necessitating the revision of the staging system (8). The most extensive changes proposed by IASLC for the eighth edition pertained to the T classification, with the importance of tumor size being highlighted (9). Moreover, invasion of the diaphragm, as an indicator of stage T4, was shown to be associated with a poor prognosis. When tumors larger than 7 cm and invasion of the diaphragm as stage T4 were included, there were statistically significant differences between T3 and T4 with respect to survival outcomes (9). These changes therefore allowed a more accurate prognosis, which could help better determine whether a patient is eligible for surgery. However, despite the advances this classification offered, we found no significant differences between the survival rate of patients with T1a and T1b disease, or between those with T1c, T2a and T2b disease. Furthermore, although the C-index and iAUC analyses indicated no statistically significant differences in the prediction of survival between the seventh and eighth editions, the increasing worse survival that occurred with increasing pathologic T stage was less apparent in the eighth edition.
We hypothesized that the discrepancies in the results of the analysis of different series was due to the diversity and origins of the database. Our study populations were different from those of the IASLC database. Although the new IASLC database for the eighth edition was collected internationally from different local databases, providing a wide geographic representation, the geographic distribution was still weighted towards Asia, especially Japan. In the eighth edition, cases from Asia contributed 79% of the clinical and pathological data to the new IASLC database, comparing only 12% of the total database in the current seventh edition (2,5). There was a predominance of early stages in Asia data in the eighth edition. However, in contrast with other Asia populations, our patients with sub-solid nodules such as AAH, AIS or minimally invasive adenocarcinoma (MIA) were not candidates for surgical resection and excluded from the study. This surgical indication contributed to a lower prevalence of early stage tumors and may skew the data toward more advanced stages in our cohort. Moreover, Rami-Porta et al. pointed out a number of limitations in their study (9). Much of the contributing database for the revision of new staging had not been designed to validate the TNM classification, nor did it always contain all of the descriptors needed for analysis. In addition, the T3 and T4 subsets in the new IASLC database were smaller, and thus a number of comparisons could not be made for theses subsets and were not reported.
Our study has some limitations. Several subgroups (T3 and T4) contained only a small number of patients, so the survival rates may have been misinterpreted. Additionally, the disease entities were different from those of the IASLC database. As mentioned above, our study had lower prevalence of early stage tumors. If we had a larger component of tumor including the bronchioloalveolar carcinoma, similar spectrum of prognostic stratification may be presented as the IASLC cohort. Furthermore, all the enrolled patients in this study had node negative status. Thus, to further assess the value of the eighth TNM staging system, evaluation of the nodal descriptors might be necessary.
In conclusion, the results of our retrospective study confirmed that the newly proposed T descriptors in the forthcoming eighth TNM staging system did not provide a more precise predictor of prognosis than those of the current seventh edition. This does not necessarily mean that the eighth edition is inferior with respect to prognostic stratification compared to the current TNM staging system. We believe that the external validation of the new TNM classification for lung cancer performed by independent groups is generally partial and prognosis should be based on local data because of different medical environments and populations.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed and approved by the Institutional Review Board at Yonsei University Health System, Severance Hospital (No. 4-2017-0935).
References
- Watanabe Y. TNM classification for lung cancer. Ann Thorac Cardiovasc Surg 2003;9:343-50. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Fukui T, Mori S, Hatooka S, et al. Prognostic evaluation based on a new TNM staging system proposed by the International Association for the Study of Lung Cancer for resected non-small cell lung cancers. J Thorac Cardiovasc Surg 2008;136:1343-48. [Crossref] [PubMed]
- Kameyama K, Takahashi M, Ohata K, et al. Evaluation of the TNM staging system proposed by the International Association for the Study of Lung Cancer at a single institution. J Thorac Cardiovasc Surg 2009;137:1180-84. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24.
- Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [Crossref] [PubMed]
- Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics 2005;61:92-105. [Crossref] [PubMed]
- Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:593-602. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.