Neoadjuvant and consolidation immuno-oncology therapy in stage III non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. Non-small cell lung cancer (NSCLC) represents 85% of newly diagnosed cases, of which 19% are localized, 24% are regional and 55% are distant (1,2). Properly staging the extent of disease at diagnosis influences the approach to treatment and prognosis. Despite ostensibly curative therapy for stage I–III NSCLC, 30–60% of patients go on to develop metastatic disease (3). Even with incremental advances in the treatment of NSCLC, the prognosis is poor: the median overall survival (OS) of metastatic NSCLC is approximately 12 months and the 5-year survival is only 1% (4). New therapies are urgently needed.
One of the most promising treatment modalities that has emerged in recent years is that of immunotherapy, specifically the use of immune checkpoint inhibitors (ICIs). These agents act on immune checkpoints that modulate the immune response, allowing for restoration of a T cell-mediated anti-tumor response. The immune checkpoint molecules in advanced clinical development include antibodies modulating cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1), and programmed death ligand 1 (PD-L1); however, many others are on the horizon. CTLA-4 is expressed on the surface of T lymphocytes and acts centrally to inhibit T cell activation by binding to the peripheral membrane protein B7 on antigen presenting cells, thereby preventing CD28 binding and stimulation of T cell activation (5). In contrast, the PD-1/PD-L1 checkpoint interaction occurs peripherally in the tumor microenvironment after T cell activation. PD-L1, expressed on the surface of tumor cells, binds to the PD-1 receptor on the T cell membrane and downregulates the immune response (6). Immunohistochemical expression of PD-L1 on tumor cell membrane and/or infiltrating immune cells is a biomarker of response to PD-(L)1 antibodies, and it is recommended that tumors from patients with newly diagnosed advanced NSCLC be tested for PD-L1 expression (7).
Multiple ICIs are currently approved in the treatment of advanced NSCLC. However, published ICI data are limited to locally advanced or metastatic disease at this time. Several phase II and III trials in advanced NSCLC have reported improved survival with PD-(L)1 antibodies, both when used alone and in combination with chemotherapy (Table 1). Collectively, the CheckMate trials, KEYNOTE trials and OAK study have led to FDA approval of nivolumab, pembrolizumab and atezolizumab, respectively, for the treatment of advanced or metastatic NSCLC. The various designs of these studies mean that anti PD-(L)1 antibodies are now indicated for untreated PD-L1 high (≥50% expression) advanced or metastatic NSCLC patients, or previously treated advanced or metastatic NSCLC patients regardless of PD-L1 status. Not only have these well-tolerated agents improved response rates and OS, but the sustained duration of benefit in some patients suggests an ongoing anti-tumor response that could be beneficial in earlier stages of NSCLC with the goal of preventing tumor recurrence (14).
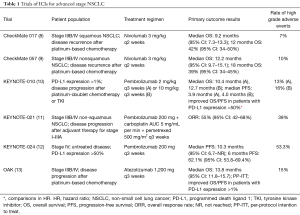
Full table
Current treatment strategies for stage III NSCLC
The current treatment for stage IIIA (T1–T4, N0–N2) NSCLC is complex and dependent on locoregional disease burden found on cross-sectional imaging and mediastinal lymph node staging. Indeed, multi-disciplinary evaluation of these patients is crucial to determine the best sequence of therapy. As the overall therapeutic goal is surgical resection for potential cure, those patients with large tumors or with certain considerations such as location or invasion require neoadjuvant chemotherapy +/− radiation if they are felt to be surgical candidates (7). Adjuvant or neoadjuvant platinum-based chemotherapy should be considered for stage III NSCLC patients who are candidates for surgery, as a 2008 meta-analysis demonstrated that adjuvant chemotherapy decreased risk of death by 5.4% over 5 years compared to surgery alone (15,16). Adjuvant concurrent or sequential radiotherapy may also be warranted in certain situations, such as resections with positive surgical margins or pathologic N2 positive nodes where neoadjuvant therapy was not administered (17).
Treatment of stage III disease involving mediastinal lymph nodes (N2) varies due to limited data. Depending on tumor size, therapeutic options include surgical resection followed by adjuvant therapy with chemotherapy or sequential chemotherapy followed by radiation, or concurrent definitive chemoradiation followed by surgical consideration depending on response. However, the use of chemotherapy or chemoradiation as a neoadjuvant treatment in certain cases of N2 disease is an area of therapeutic complexity. The data are unclear about the survival benefit of surgery after neoadjuvant treatment compared to definitive chemoradiation; earlier phase II studies reported a potential benefit of surgery, but subsequent randomized phase III studies have not observed the same benefit (18-22). In the 2009 phase III study comparing concurrent chemoradiotherapy followed by surgery to concurrent chemoradiotherapy followed by continued radiotherapy, the median OS was 23 and 22 months, respectively (18). It should be noted that the treatment approach for poor-risk patients—those who exhibit poor prognostic features established in risk assessment models—with resectable stage III NSCLC who are not candidates for surgery or concurrent chemoradiation is less well defined. Definitive radiotherapy is currently a viable option, and studies have shown that using radiotherapy alone yielded a median survival of approximately 29 months (23).
Concurrent definitive chemoradiation has been established as the standard of care for unresectable stage IIIB (N3 disease) NSCLC, with multiple studies showing a median OS of around 17 months (24,25).
The current therapeutic options for stage III disease are complex, and currently include surgery, radiation and classical cytotoxic chemotherapy. The long-term response and survival in these patients with current standard therapies however, is overall poor with a rate of 36% and 19% at 5 years for stages IIIA and IIIB, respectively (26).
Survival/relapse risk for resectable disease: defining the unmet need
Well tolerated, effective neoadjuvant and adjuvant therapies for resectable lung cancer are therefore urgently needed. Even after curative resection of NSCLC, approximately 60% of patients with stage IIIA disease develop recurrence after 3 years (27). As discussed, both neoadjuvant and adjuvant chemotherapy have been shown to improve survival in resectable NSCLC (28,29). However, each option has its respective limitations. Neoadjuvant chemotherapy can delay surgery if treatment-related toxicities arise, increase complications during surgery, and in some cases possibly prevent surgical resection through tumor progression (30). Difficulties from adjuvant chemotherapy arise from treatment delays due to patient recovery after surgery and consequent poor compliance (31,32). Immunotherapy may fulfill this unmet need by providing a better tolerated treatment option when compared with conventional chemotherapy, while limiting the impact of treatment-related toxicities on surgical resection.
Preclinical rationale for neoadjuvant immunotherapy
A recent study published in the preclinical setting supports the rationale for administering immunotherapy as a neoadjuvant treatment (33). Using an immunocompetent murine model of triple negative breast cancer (TNBC), it was demonstrated that mice treated with neoadjuvant anti-PD-1/anti-CD137 combination had a 100-day survival of 50% compared to 0% in mice treated with adjuvant anti-PD-1/CD137. The improved efficacy of neoadjuvant treatment was also demonstrated on a cellular level. Neoadjuvant anti-PD-1/anti-CD137 resulted in a sustained increase of tumor-specific CD8+ T cells in the blood even after the tumor had been removed; adjuvant anti-PD-1/anti-CD137 showed a significantly lower increase in CD8+ T cell percentage in blood (6% to 1.1%, P=0.0263). Levels of these tumor-specific CD8+ cells predicted long-term survival, with the majority of mice with high CD8+ levels surviving longer than 100 days (33). In addition, the authors also report that depleting T cells and natural killer cells in long-term survivors did not reduce survival as would be expected if the tumor was merely dormant, suggesting permanent tumor kill with neoadjuvant therapy.
Neoadjuvant immunotherapy in other tumor types
The success of neoadjuvant immunotherapy in treating other cancers provides additional rationale for its use in stage III NSCLC. Currently established immunotherapies in advanced NSCLC first showed potential in treating melanoma patients, and preliminary results from neoadjuvant immunotherapy melanoma trials have been recently presented. The phase I OpACIN trial administered neoadjuvant nivolumab and ipilimumab in ten stage III melanoma patients and reported three complete responders and five partial responders, all of whom had not relapsed at a median follow-up of 45 weeks (34). Similarly, in a pooled clinical analysis of four ongoing clinical trials (NCT02437279, NCT02231775, NCT02519322, NCT01972347) from the International Neoadjuvant Melanoma Consortium, 21 patients received either neoadjuvant combined nivolumab/ipilimumab or nivolumab alone; eight complete responses with no recurrence were observed, and only three of the 13 remaining patients had recurring disease after neoadjuvant immunotherapy with surgery (35). The benefit of ipilimumab as neoadjuvant therapy has also been reported in case studies (36,37).
For locally advanced TNBC, the phase Ib KEYNOTE-173 study is examining combination pembrolizumab and chemotherapy in the neoadjuvant setting. Patients were assigned to either neoadjuvant pembrolizumab with nab-paclitaxel or pembrolizumab with nab-paclitaxel and carboplatin. With ten patients on each arm, interim data showed pathologic complete response (pCR) rates of 50% and 80%, respectively (38).
Neoadjuvant and adjuvant immune checkpoint inhibition in NSCLC: currently available data
Interim results from a phase II trial testing neoadjuvant nivolumab in newly diagnosed resectable Stage IA–IIIA NSCLC (NCT02259621) were presented at the 2016 ESMO Congress and updated at the 2017 ASCO Annual Meeting. This study planned to administer two doses of nivolumab over 4 weeks prior to planned surgical resection. From 22 enrolled patients, 21 were deemed eligible and treated with neoadjuvant nivolumab and of these, 20 patients underwent surgical resection. The primary endpoints of feasibility and safety were met: administering nivolumab did not delay surgery, and treatment was well tolerated with one treatment-related grade 3 toxicity and no treatment-related grade 4 or 5 toxicities. Major pathologic response (MPR) was set as a secondary outcome and is defined as ≤10% residual viable tumor in the primary tumor. MPR captures treatment-specific anti-tumor activity and has been established as a potential surrogate of OS (39,40), Among the 21 per protocol patients 18 patients (85%) demonstrated stable disease, and 2 patients (10%) demonstrated a partial response while one patient had progressive disease. Notably, CT imaging underestimated the extent of nivolumab response, as MPR was reported in 9/21 cases (43%, 95% CI: 24–63%) (40). With a median follow-up of 12 months, 2/20 resected patients had experienced recurrence (41). Based on these encouraging data, this trial has expanded to examine combination neoadjuvant therapy with nivolumab and ipilimumab.
Durvalumab (anti-PD-L1) is now an FDA-approved option in unresectable stage III NSCLC. The phase III placebo-controlled PACIFIC trial (NCT02125461) (42) tested durvalumab after standard chemoradiotherapy and demonstrated a significant improvement in progression-free survival (PFS) (43).
Similarly, atezolizumab is being tested in the phase II DETERRED trial (NCT02525757) in patients with unresectable stage III NSCLC. In this study, atezolizumab is administered either concurrently with standard chemoradiation therapy followed by additional doses of atezolizumab during a consolidation phase, or solely during the consolidation phase after standard chemoradiation. The primary outcome is safety with a secondary outcome of PFS. Recently reported interim results of the consolidation only arm revealed a 20% (2/10) rate of high-grade immune-related adverse events. One patient who experienced grade 3 COPD exacerbation discontinued treatment after one dose of atezolizumab. Of the other 9 patients, 3 patients (30%) progressed after 6, 8, and 14 doses; the remaining patients remained on treatment at the time of report (44).
Neoadjuvant immune checkpoint inhibition in NSCLC: ongoing trials
A comprehensive list of ongoing trials investigating the use of ICIs in the neoadjuvant treatment of stage III NSCLC can be found in Table 2.
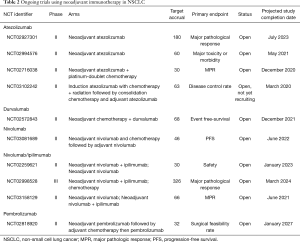
Full table
In addition to the neoadjuvant nivolumab study with interim results mentioned earlier, two other trials are currently studying combination nivolumab with ipilimumab (anti-CTLA-4) in the neoadjuvant setting. CheckMate 816 is a randomized, open label phase III trial (NCT02998528) comparing neoadjuvant combination nivolumab and ipilimumab or platinum doublet plus nivolumab versus standard neoadjuvant platinum doublet chemotherapy in stage IB (≥4 cm)–IIIA NSCLC. The rationale for combination nivolumab and ipilimumab in CheckMate 816 is based on results from CheckMate 012, a phase I study in stage IIIB/IV NSCLC patients that demonstrated a higher objective response rate and PFS with combination therapy compared to nivolumab monotherapy (45). Similarly, chemotherapy-anti-PD-1 combination therapy has demonstrated promising efficacy in advanced NSCLC (11). The primary endpoint of this study is pCR rate measured at the time of surgery. The efficacy of combination nivolumab and ipilimumab compared to nivolumab monotherapy for stage I-IIIA NSCLC in the neoadjuvant setting is being evaluated in the NEOSTAR trial (NCT03158129).
TOP 1501 (NCT02818920), a phase II trial, is evaluating the surgical feasibility rate in patients following neoadjuvant pembrolizumab and also includes consolidation pembrolizumab following adjuvant therapy. Other adjuvant trials look to increase the versatility of pembrolizumab as a therapeutic option.
Atezolizumab is being studied in many ongoing clinical trials in the neoadjuvant setting. Neoadjuvant atezolizumab is in phase II testing, and two trials are administering atezolizumab as monotherapy. The PRINCEPS trial (NCT02994576) gives patients one dose of neoadjuvant atezolizumab, while another trial (NCT02927301) gives two neoadjuvant doses and then follows adjuvant chemotherapy with potential consolidation atezolizumab for up to 1 year (46).
As previously mentioned, durvalumab has not yet been the Food and Drug Administration (FDA)-approved for treatment of NSCLC. One major clinical trial outside the US, however, is investigating durvalumab as both a neoadjuvant and adjuvant therapy. This phase II study in Switzerland combines durvalumab with neoadjuvant chemotherapy followed by adjuvant durvalumab in patients with stage IIIA disease (NCT02572843). In the US, two phase III trials of durvalumab in stage III NSCLC are currently open. As mentioned previously, results from the PACIFIC trial in unresectable stage III NSCLC have demonstrated promising efficacy (43).
Adjuvant immune checkpoint inhibition in NSCLC: ongoing trials
Ongoing trials investigating the use of ICIs in the adjuvant setting of stage III NSCLC can be found in Table 3. With many of these studies, along with the neoadjuvant studies mentioned earlier, set to be completed in the next 3–5 years, the body of evidence supporting the use of immunotherapy for early stage NSCLC may grow.
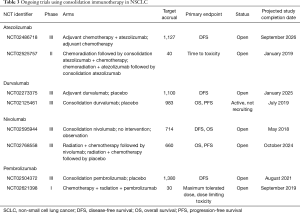
Full table
Nivolumab is being studied in the adjuvant and consolidation settings in two phase III trials. The ANVIL trial (NCT02595944) is studying adjuvant nivolumab after surgical resection, and enrollment is ongoing (47). In the consolidation setting, nivolumab is also being compared against placebo in patients with unresectable stage III NSCLC after receiving chemoradiation (NCT02768558). Along with the previously mentioned neoadjuvant nivolumab/ipilimumab trials, the results of these studies may lead to immunotherapy indications for early stage NSCLC.
Pembrolizumab is being evaluated in the adjuvant treatment of early stage NSCLC. The phase III PEARLS trial (NCT02504372) compares efficacy of pembrolizumab to placebo following surgical resection and standard adjuvant therapy. A phase I trial is examining the maximum tolerated dose and safety of pembrolizumab when combined with concurrent chemoradiation therapy in unresectable stage III disease (NCT02621398).
Also, in patients with unresectable stage III NSCLC, Alliance Foundation Trials is administering induction atezolizumab with chemoradiotherapy, followed by consolidation chemotherapy with adjuvant atezolizumab (NCT03102242). The phase III IMpower010 trial (NCT02486718), focuses on consolidation atezolizumab compared to placebo following adjuvant chemotherapy (48).
Finally, IONESCO (NCT02273375) is a phase III study of adjuvant durvalumab compared to placebo in completely resected NSCLC.
Conclusions
The numerous ongoing clinical trials of ICIs in the neoadjuvant and adjuvant settings demonstrate the promising future of immunotherapy in the complex management paradigm of stage III NSCLC. Even though currently available data are limited, the interim reports suggest that perioperative use of PD-(L)1 antibodies is well tolerated and may have efficacy. For unresectable stage III NSCLC, the PACIFIC trial have met its primary endpoint of improved PFS with durvalumab, with recent FDA approval in this treatment setting. In addition to nivolumab, atezolizumab is being studied as neoadjuvant therapy; four phase II studies are currently open with results to be released over the next 3–6 years. With multiple trials investigating each of these ICIs in large patient cohorts, we eagerly await the results of these studies to determine their role in the management of stage III NSCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review (CSR), 1975-2014. Bethesda: National Cancer Institute, 2017.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Boyd JA, Hubbs JL, Kim DW, et al. Timing of local and distant failure in resected lung cancer: implications for reported rates of local failure. J Thorac Oncol 2010;5:211-4. [Crossref] [PubMed]
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [Crossref] [PubMed]
- Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 2011;11:852-63. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- NCCN. Non-Small Cell Lung Cancer (Version 7. 2017) 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemo-therapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, mul-ticentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Brahmer J, Horn L, Jackman D, et al. Five-year follow-up from the CA209-003 study of nivolumab in previously treated advanced non-small cell lung cancer (NSCLC): Clinical characteristics of long-term survivors. Washington: 2017 AACR Annual Meeting, 2017.
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 2007;25:5506-18. [Crossref] [PubMed]
- Wang EH, Corso CD, Rutter CE, et al. Postoperative Radiation Therapy Is Associated With Improved Overall Survival in Incompletely Resected Stage II and III Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2727-34. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III ran-domised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Burkes RL, Shepherd FA, Blackstein ME, et al. Induction chemotherapy with mito-mycin, vindesine, and cisplatin for stage IIIA (T1-3, N2) unresectable non-small-cell lung cancer: final results of the Toronto phase II trial. Lung Cancer 2005;47:103-9. [Crossref] [PubMed]
- Eberhardt WE, Pottgen C, Gauler TC, et al. Phase III Study of Surgery Versus De-finitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemo-therapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194-201. [Crossref] [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Weder W, Collaud S, Eberhardt WE, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg 2010;139:1424-30. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus se-quential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692-9. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Qiang G, Guo Y, Xiao F, et al. Analyses of risk factors for postoperative recurrence after curative resection of stage III A-N2 non-small cell lung cancer. Zhonghua Yi Xue Za Zhi 2014;94:3239-43. [PubMed]
- NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Song WA, Zhou NK, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. J Thorac Oncol 2010;5:510-6. [Crossref] [PubMed]
- Salva F, Felip E. Neoadjuvant chemotherapy in early-stage non-small cell lung cancer. Transl Lung Cancer Res 2013;2:398-402. [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res 2014;3:242-9. [PubMed]
- Liu J, Blake SJ, Yong MC, et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov 2016;6:1382-99. [Crossref] [PubMed]
- Rozeman EA, Blank CU, Van Akkooi AC, et al. Neoadjuvant ipilimumab + nivolumab (IPI+NIVO) in palpable stage III melanoma: Updated data from the OpACIN trial and first immunological analyses. Chicago: 2017 ASCO Annual Meeting, 2017.
- Menzies AM, Rozeman EA, Amaria RN, et al. Preliminary results from the international neoadjuvant melanoma consortium (INMC). Chicago: 2017 ASCO Annual Meeting, 2017.
- Howie LJ, Tyler DS, Salama AK. Neoadjuvant use of ipilimumab in locally advanced melanoma. J Surg Oncol 2015;112:841-3. [Crossref] [PubMed]
- Laks S, Brueske KA, Hsueh EC. Neoadjuvant treatment of melanoma: case reports and review. Exp Hematol Oncol 2013;2:30. [Crossref] [PubMed]
- Schmid P, Park YH, Muñoz-Couselo E, et al. Pembrolizumab (pembro) + chemotherapy (chemo) as neoadjuvant treatment for triple negative breast cancer (TNBC): Preliminary results from KEYNOTE-173. Chicago: 2017 ASCO Annual Meeting, 2017.
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Chaft JE, Forde PM, Smith KN, et al. Neoadjuvant nivolumab in early-stage, resectable non-small cell lung cancers. Chicago: 2017 ASCO Annual Meeting, 2017.
- FDA expands approval of Imfinzi to reduce the risk of non-small cell lung cancer progressing. Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm597217.htm
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Lin SH, Lin Y, Price J, et al. DETERRED: PD-L1 blockade to evaluate the safety of lung cancer therapy using carboplatin, paclitaxel, and radiation combined with MPDL3280A (atezolizumab). Chicago: 2017 ASCO Annual Meeting, 2017.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Owen DH, Bunn PA, Johnson BE, et al. A phase II study of atezolizumab as neoad-juvant and adjuvant therapy in patients (pts) with resectable non-small cell lung cancer (NSCLC). Chicago: 2017 ASCO Annual Meeting, 2017.
- Chaft JE, Dahlberg SE, Gerber DE, et al. EA5142 adjuvant nivolumab in resected lung cancers (ANVIL): The newest study in the ALCHEMIST platform. Chicago: 2017 ASCO Annual Meeting, 2017.
- Wakelee HA, Altorki NK, Vallieres E, et al. A phase III trial to compare atezolizumab (atezo) vs best supportive care (BSC) following adjuvant chemotherapy in patients (pts) with completely resected NSCLC: IMpower010. Chicago: 2017 ASCO Annual Meeting, 2017.


