Comparative study of systematic thoracoscopic lymphadenectomy and conventional thoracotomy in resectable non-small cell lung cancer
Introduction
Lung cancer is a serious hazard to human health and life, with a significant rising trend in terms of morbidity and mortality around the world in recent years. This condition has become the leading cause of morbidity and mortality worldwide, both for developed and developing countries (1). Although there are many methods for treating lung cancer at present, the recognized option of choice for the treatment of early- and mid-stage non-small cell lung cancer (NSCLC) is surgical excision, and the standard surgical method is lobectomy combined with systematic lymph node dissection. As early as in 1983, Martini et al. (2) first reported the use of lobectomy and mediastinal lymph node dissection for the treatment of primary lung cancer.
With the wide application of minimally invasive techniques in the surgical field, the use of video-assisted thoracoscopy surgery (VATS) in the treatment of NSCLC has been increasingly valued by thoracic surgeons. With the greatest advantage of minimal invasiveness, reduced postoperative pain and less damage to the respiratory muscle and pulmonary function, the VATS technique has been applied in the lobectomy of lung cancer as early as in 1992 (3). In 1995, McKenna et al. (4) first reported the use of VATS lobectomy combined with mediastinal lymph node dissection in the treatment of primary lung cancer.
Thorough lymph node dissection is one of the keys for successful comprehensive treatment of lung cancer, as it provides definite staging and guidance for the prognosis and the next treatment, and can improve the local remission rate and prolong disease-free survival time. According to the guidelines issued by the European-Society of Thoracic Surgeons (ESTS), systematic lymph node dissection is required for resectable NSCLC regardless of VATS or thoracotomy (5). Whether VATS allows thorough mediastinal lymph node dissection and can achieve comparable effects to thoracotomy has been controversial. At present, the reported results varied in different studies on the use of VATS for lobectomy combined with lymphadenectomy of resectable NSCLC compared with thoracotomy (6-14). So far, however, the number of studies comparing the two techniques is not large enough for a comprehensive assessment of the effectiveness and safety of systematic lymphadenectomy using VATS versus thoracotomy. This study aims to determine the effectiveness and safety of VATS-based systematic lymphadenectomy by retrospectively analyzing the related multi-center, large-scale clinical data.
Materials and methods
Clinical data
The clinical data of patients with NSCLC who underwent VATS or thoracotomy combined with lobectomy and systematic lymphadenectomy in eight hospitals in China from January 2001 to January 2008 were retrospectively analyzed, and 5,620 patients were included in this study. Upon enrollment, all participants were engaged in a series of preparation before surgery, including quitting smoking, respiratory function exercise, administration of phlegm drugs and chest physiotherapy.
Preoperative examination and surgical methods
Before surgery, all participants received physical examination, routine blood tests, ECG, cardiac color Doppler ultrasound and lower extremity deep venous color Doppler ultrasound. Respiratory function tests included pulmonary ventilation-dispersion function tests. Coronary artery CT or treadmill activity tests were performed in patients with suspected coronary heart disease over the age of 60, as well as coronary interventional examination, if necessary.
Preoperative tumor staging was based mainly on chest CT, head and abdominal MRI, whole body bone scan, and bronchoscopy. PET/CT scans were recommended for patients considered to be stage II or above.
All participants underwent VATS or open chest lobectomy and hilar and mediastinal lymph node dissection, of which the specific surgical techniques were already reported in our previous study (15).
Thoracotomy group: a standard posterolateral incision of about 10-20 cm was made for placement of intercostal distraction to carry out the thoracotomy under direct vision. The operation included anatomic lobectomy plus systematic mediastinal lymph node dissection.
Systematic mediastinal lymph node dissection was common in both procedures, instead of lymph node sampling, involving at least three groups of mediastinal and intrapulmonary lymph nodes (including subcarinal lymph nodes). The surrounding fat tissue was be resected together with the lymph nodes en bloc. The resected lymph node specimens were independently examined and interpreted by two or more senior pathologists.
Data collection and follow-up
The demographic data, operative time, blood loss, number of dissected lymph nodes, postoperative hospital stay, postoperative chest tube duration, postoperative tumor type, stage, and occurrence of postoperative chylothorax were collected for all patients.
Statistical analysis
Measurement data were expressed as mean ± standard deviation (). The statistical analysis was completed in SPSS 13, with P<0.05 indicating a statistically significant difference.
Results
Clinical data
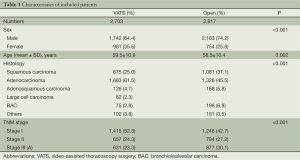
A total of 5,620 patients were finally included in the retrospective study, with 2,703 in the VATS group, including 1,742 men (64.4%) and 961 women (35.6%), aged 59.5±10.9 years; and 2,917 in the thoracotomy group, including 2,163 men (74.2%), and 754 women (25.8%), aged 58.5±10.4 years (Table 1).
Full table
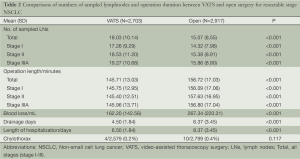
All patients underwent VATS or open chest lobectomy plus systematic lymphadenectomy. Comparing the VATS with the thoracotomy groups, the mean operative time was 146 vs. 157 min, with a significant difference (P<0.001); and the average blood loss was 162 vs. 267 mL, with a significant difference (P<0.001) (Table 2). The postoperative pathological test showed 1,663 patients with adenocarcinoma (61.5%), 675 patients with squamous cell carcinoma (25.0%), 126 patients with adenosquamous carcinoma (4.7%), and 239 patients with other types of tumors (8.9%) in the VATS group; and 1,326 patients with adenocarcinoma (45.5%), 1,081 patients with squamous cell carcinoma (37.1%), 168 patients with adenosquamous carcinoma (5.8%), and 342 patients with other types of tumors (11.8%) in the thoracotomy group (Table 1). According to the 2009 International Association for the Study of Lung Cancer (IASLC) staging criteria (16), all patients were subject to clinical pathological staging classification. There were 1,415 patients at stage I (52.3%), 657 patients at stage II (24.3%), and 631 patients at stage IIIA (23.3%) in the VATS group; and 1,246 patients at stage I (42.7%), 794 patients at stage II (27.2%), and 877 patients at stage IIIA (30.1%) in the thoracotomy group (Table 2).
Full table
Postoperative conditions (Table 2)
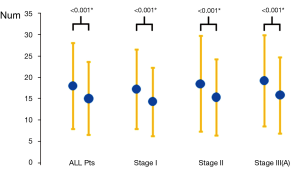
Comparing the two groups of patients data, the number of lymph node dissection (Figure 1): 18.03 in the VATS group and 15.07 in the thoracotomy group on average, with a significant difference (P<0.001); blood loss: 162.2 mL in the VATS group and 267.34 mL in the thoracotomy group on average, with a significant difference (P<0.001); postoperative drainage time: 4.5 days in the VATS group and 6.37 days in the thoracotomy group on average, with a significant difference (P<0.001); postoperative hospital stay: 6.5 days in the VATS group and 8.37 days in the thoracotomy group on average, with a significant difference (P<0.001); proportion of postoperative chylothorax: 0.2% (4/2,579) in the VATS group and 0.4% (10/2,799) in the thoracotomy group, without significant difference (P>0.05).
Discussion
Lymph node metastasis is an important way of local and distant metastases in malignant cancer, as well as in NSCLC. It has a very important role in the prognostic determination and development of therapeutic strategies. Thus, for resectable NSCLC, the standard surgical method is lobectomy in combination with systematic lymph node dissection, which can improve the local control rate and prolong disease-free survival time.
Although it remains unconfirmed whether systematic lymphadenectomy can benefit patients with NSCLC oncologically, accurate lymph node staging still plays an important role in determining the need of postoperative adjuvant therapy and prognosis. Studies have shown that systematic lymphadenectomy is significantly superior to lymph node sampling in accurate staging. Investigators have found 4% patients at N2 stage with systematic lymph node dissection from 524 stage I patients who were identified with negative lymph nodes based on the sampling (17).
In the past, standard posterior lateral open chest lobectomy and lymph node dissection was mostly used for early and mid-stage resectable NSCLC. However, it is associated with a surgical incision often larger than 10 cm, extensive injury, slower postoperative recovery and higher incidence of postoperative complications. Since the early 1990s, VATS has been rapidly developed and widely applied in the world, involving almost all areas of general thoracic surgery. Compared with thoracotomy, VATS enables a smaller incision without removing or stretching the ribs open, sparing respiratory muscles from injures and thus minimizing the loss of lung function. Moreover, with a smaller incision, patients will suffer less pain postoperatively and expectorate more easily, reducing the incidence of postoperative pulmonary infection and complications as well.
The safety and effectiveness of VATS lobectomy combined with lymph node dissection for the treatment of early NSCLC has been confirmed, more and more studies have shown that this technique has comparable long-term oncological outcomes as a radical option to traditional open thoracic surgery (18,19). Moreover, National Comprehensive Cancer Network (NCCN) treatment guidelines for NSCLC has also clarified that VATS is a viable option for treating resectable lung cancer, particularly for those who can not tolerate standard thoracotomy due to physical conditions. This means that VATS treatment of NSCLC has covered most internationally recognized indications for surgical treatment of lung cancer.
As we all know, a thorough lymph node dissection is essential for the prognosis of patients with NSCLC, but it remains controversial whether this can be achieved with thoracoscopic systematic lymphadenectomy for NSCLC. In contrast to the thoracic surgery, many surgeons suspect the feasibility and thoroughness of thoracoscopic lymph node dissection. The primary concern is residual lymph nodes. In this regard, many studies have confirmed that after VATS lymph node dissection, the residual lymph node rate is very low. Hoksch et al. (20) did VATS lymphadenectomy in corpses followed by standard lateral open chest exploration, and the results showed no significant residual hilar and mediastinal lymph nodes. Sagawa et al. (21) performed VATS lymph node dissection in 29 NSCLC stage I patients followed by open chest exploration, and confirmed that there were only 2-3% of residues.
Since it has been applied in lymph node dissection, VATS has witnessed numerous controversies about whether it is superior or inferior to thoracotomy in this regard. Retrospective or prospective clinical studies yielded varying results as well (6-14,22). Ramos et al. (11) conducted a retrospective study to compare the number of dissected lymph nodes and stations with the two approaches by collecting the clinical and pathological data from patients with stage I non-small cell lung cancer patients. The results showed that an average dissection number of 5.1 stations in the VATS group, which was more than 4.5 stations in the open chest group, with a significant difference. However, the average number of 22.6 dissected nodes in the VATS group was far fewer than 25.4 nodes in the open chest group, with a significant difference. Lee et al. (23) analyzed 141 VATS patients and 115 cases of thoracic surgery for resectable NSCLC, finding that VATS yielded fewer dissected nodes compared with the open chest group (11.3±6.4 vs. 14.3±8.8, P=0.001), and the total number of dissected stations (3.1±1.1 vs. 3.8±1.2, P<0.001). Further analysis revealed that both differences came mainly from the dissection of mediastinal lymph nodes. On the other hand, some studies have confirmed that there is no difference in the number of either dissected nodes or dissected stations between the two approaches. Yang et al. (22) compared 62 patients with resectable NSCLC, which 31 cases in each of the VATS and thoracotomy groups, and found no significant difference in the number of either node or station dissected. In the present study, we found through statistical analysis that there was a mean number of dissected nodes of 18.03 in the VATS group and 15.07 in the thoracotomy group, with a significant difference (P<0.001) between the two groups, which is inconsistent with previous reports. We believe that the thoracoscopic vision has almost zero dead angles during intrathoracic operations. It can provide a good surgical field and has a visual zoom effect to magnify the surgical field, with which the hilar structures and mediastinal lymph node stations can be more clearly identified and exposed. In this way, we are able to clean out more mediastinal lymph node, reducing the incidence of residual lymph nodes.
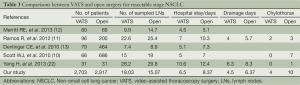
The safety of VATS lobectomy in combination with systematic lymphadenectomy for resectable NSCLC is another concern. We have found through literature review and comparison (Table 3) that the majority of studies suggest that VATS has great advantages in terms of postoperative complications, postoperative chest tube drainage duration and postoperative hospital stay compared with thoracotomy. This study also confirms this conclusion. We believe that the smaller surgical wound and more clearly exposed blood vessels, lymph nodes and lymph vessels during VATS have made it possible to accurately dissect target tissue during dissection without damaging small blood vessels and lymph nodes, thus reducing lymphatic drainage and the occurrence of postoperative chylothorax, allowing earlier postoperative extubation and reduced postoperative hospital stay.
Full table
However, there are several limitations in this study due to its retrospective nature. Although this study has involved the most cases in comparison of VATS and open chest lymph node dissection, the origination of data from several studies with surgeons of varying thoracoscopic technical levels may have contributed to certain data deviation. Secondly, this study only analyzes two surgical procedures only in terms of the number of lymph node dissection and related postoperative complications, without comparing the differences in the prognosis. Therefore, a more comprehensive prospective study will be needed to further determine the safety and effectiveness of VATS lymph node dissection.
In conclusion, for patients with resectable NSCLC, VATS systematic lymph node dissection is safe and effective with acceptably low incidences of postoperative complications, and significantly faster postoperative recovery compared with traditional open chest surgery.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Martini N, Flehinger BJ, Nagasaki F, et al. Prognostic significance of N1 disease in carcinoma of the lung. J Thorac Cardiovasc Surg 1983;86:646-53. [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- McKenna R Jr. Vats lobectomy with mediastinal lymph node sampling or dissection. Chest Surg Clin N Am 1995;5:223-32. [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg 2010;89:S2107-11. [PubMed]
- Watanabe A, Mishina T, Ohori S, et al. Is video-assisted thoracoscopic surgery a feasible approach for clinical N0 and postoperatively pathological N2 non-small cell lung cancer? Eur J Cardiothorac Surg 2008;33:812-8. [PubMed]
- Kim HK, Choi YS, Kim J, et al. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:1288-93. [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [PubMed]
- Ramos R, Girard P, Masuet C, et al. Mediastinal lymph node dissection in early-stage non-small cell lung cancer: totally thoracoscopic vs thoracotomy. Eur J Cardiothorac Surg 2012;41:1342-8; discussion 1348. [PubMed]
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [PubMed]
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5; discussion 1736.
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [PubMed]
- He J, Shao W, Cao C, et al. Long-term outcome and cost-effectiveness of complete versus assisted video-assisted thoracic surgery for non-small cell lung cancer. J Surg Oncol 2011;104:162-8. [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest 2011;139:1124-9. [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [PubMed]
- Hoksch B, Ablassmaler B, Walter M, et al. Radical thoracoscopic lobectomy with lymphadenectomy in a cadaver model. Can J Surg 2002;45:376-80. [PubMed]
- Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by video-assisted thoracic surgery: can it be perfect? Ann Thorac Surg 2002;73:900-4. [PubMed]
- Yang H, Li XD, Lai RC, et al. Complete mediastinal lymph node dissection in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Thorac Cardiovasc Surg 2013;61:116-23. [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 960-1. [PubMed]


