Catheter-based alternative treatment for early-stage lung cancer with a high-risk for morbidity
Introduction
Surgical resection remains the first-line treatment for early-stage non-small cell lung cancer (NSCLC) (1). Although the development of minimally invasive video-assisted thoracic surgery (VATS) and other interventions have allowed sicker patients to undergo surgical resection (2,3), nevertheless, approximately one-fifth of patients with early-stage NSCLC cannot tolerate pulmonary resection or less invasive forms, such as wedge resection or segmentectomy (4), due to additional comorbidities and limited cardiopulmonary reserve (1,5). Non-surgical options for these medically inoperative patients have emerged rapidly and stereotactic body radiation therapy (SBRT) has shown promising results for inoperable stage I NSCLC (6). However, this technique could be limited for central tumours or those adjacent to the chest wall because of greater chances for significant complications, such as major pulmonary haemorrhage, fibrosis (grade ≥3) or chest wall pain.
The goal of the current article is to review other catheter-based non-surgical treatments for early-stage NSCLC, including radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation (CRA) and photodynamic therapy (PDT). The role of SBRT is not discussed in this article.
RFA
RFA is usually implemented under computed tomography (CT) or ultrasound guidance depending on the location and size of the lesion. The therapeutic mechanism involves introducing a thermal effect to a tumour via electromagnetic energy (7). A high-frequency electrical current produced by a radiofrequency generator moves from an active electrode inserted within the lesion to a dispersive electrode placed on the patient, which generates heat and coagulates tissue; a temperature >60 °C within the tumour leads to cell death via protein denaturation and coagulation necrosis. Studies have shown that most probes on the market induce tissue necrosis up to 5 cm, which makes this method capable of treating small to moderate size tumours (8).
In most of the reported series, RFA requires conscious sedation or general anaesthesia, and at least 24 hours post-procedural observation is needed. Inevitably, one can expect some surrounding normal tissue to be damaged during the RFA procedure. However, normal lung parenchyma can be partly protected due to the presence of air providing an insulating effect to the thermal energy. The somewhat opposite of this powerful phenomenon may hamper the utility of this technique when applied to a tumour near vascular structures. The “heat sink effect”, which states that vessels >3 mm in diameter that are closely related to the tumour reduce the amount of energy delivered to the lesion because of heat convection with adjacent circulation, thus limit RFA utilization in specific target areas.
The efficacy of RFA for treating stage I NSCLC has been examined in a series of studies. Most of these reports tended to be from single centres and had follow-ups of about 2 years (Table 1) (9-15). The 1-year survival rates were impressive at 78–95% across publications, but 3-year survival declined to 36–74%. Ambrogi et al. reported one of the largest cohorts for RFA treatment of 57 stage I NSCLC with a mean follow-up of 47 months (13). The complete response (CR) rate was 65.9% for stage IA and 40% for stage IB tumours. Mean local recurrence interval was 25.9 months, indicating acceptable local control. Cancer-specific survival was 89% at 1 year, 59% at 3 years and 40% at 5 years. Median survival was 83% at 1 year, 40% at 3 years and 25% at 5 years.

Full table
The ACSOG Z4033 trial (14), which enrolled 51 patients with stage IA NSCLC from 16 centres, reported that RFA therapy did not impair pulmonary function and resulted in only grades 1–3 adverse events in the first 3 months following ablation. The local tumour recurrence-free rate was 68.9% at 1 year and 59.8% at 2 years. Interestingly, patients treated with RFA were able to tolerate other local therapies, as 19 of the relapsed patients received either repeat thermal ablation or SBRT after local recurrence.
MWA
MWA is considered superior to other options because it allows continuous and uniform delivery of heat through different tissues and achieves longer ablation times. MWA is not limited by the heat sink effect, pacemaker interference, or the need for grounding pads to be placed on the patient’s skin surface (16). After introducing the probe into the tumour percutaneously, electromagnetic waves excite and oscillate water molecules, thus resulting in thermocoagulation of the tissue surrounding the probe. Improved energy deposition generates a spherical rather than irregular ablation zone (up to 2 cm from the probe tip) in a shorter time than RFA (17). Intratumoural temperatures can be monitored through a separate antenna located adjacent to the MWA probe. Moreover, multiple probes can be placed simultaneously to increase ablation volume.
Less clinical data are available regarding the effectiveness of MWA in the treatment of early-stage NSCLC compared with those of RFA. Han et al. (18) reviewed the results of MWA for treating 28 patients >75 years of age with stage I or lymph node-negative stage IIA. Interestingly, the local recurrence rate was 32.1%, which is similar to reports of RFA under a median follow-up time of 22.5 months. Median time to local progression was 28.0 months. The 1-, 2-, 3- and 4-year overall survival rates were 91.7%, 76.5%, 47.9% and 47.9%, while the cancer-specific survival rates were 94.7%, 73.9%, 64.7% and 64.7%, respectively. No significant side-effects or complications were observed. Acksteiner et al. (19) reported their experience using MWA in 10 patients ≥75 years of age with early-stage NSCLC. With a median follow-up of 12 months, only one patient died 19 months after ablation, with local recurrence at the treatment site. The authors also reported the successful use of re-ablations in two patients due to positive positron emission tomography-CT findings with the first ablation.
CRA
CRA utilises cold temperatures rather than heat and is based on the Joules-Thompson effect with alternating freeze/thaw cycles. Argon exposure causes temperatures as low as −150 °C upon transition from a liquid to a gaseous state. Rapid freezing leads to ice crystal formation both intra- and extracellularly, which disrupts cellular processes. Additionally, vasoconstriction and local tissue oedema result in hypoxic tissue damage and coagulative necrosis. The freeze cycle is alternated with a thaw cycle by introducing high-pressure helium gas to raise the temperature to 20–40 °C.
The temperature should be at least −20 °C to achieve lethal effects in cells, although this has not been demonstrated in vivo (20). A 2–3-mm-diameter probe can produce a freeze area of 2–3 cm in diameter and 4 cm in length. Similar to MWA, the probe temperature can be measured with an additional needle. The diameter/number of probes and the number of freeze/thaw cycles (two or three in most cases) depends on tumour size and location. Successful ablation usually requires a cryozone to be approximately 1 cm beyond the radiographically designated tumour region. Notably, cryotherapy also suffers from the heat/cold sink effect that is observed in RFA. CRA may have the theoretical advantage for treating centrally located tumours due to the relative resistance of collagenous architecture to freeze thawing, allowing for minimisation of damage to adjacent important structures.
There are few reports on cryotherapy with an emphasis on stage I NSCLC. Yamauchi et al. (21) retrospectively reviewed the results of 34 tumours with a mean diameter of 1.4 cm with histologically proven stage I lung cancers. Local tumour progression was noticed in only one tumour after a median follow-up of 23 months. Median overall survival was 68 months with 2- and 3-year disease-free survival rates of 78% and 67%, respectively. In another study, Moore et al. (22) investigated cryotherapy in 45 patients with a low forced expiratory volume in 1 s of 1.2 L. Mean tumour diameter was 1.9 cm, and the number of probes per unit tumour diameter was 1.4 probes/cm. The combined local and regional recurrence rate was 36.2%. The 5-year overall survival, cancer-specific survival and progression-free survival rates were 67.8%, 56.6% and 87.9%, respectively.
PDT
In PDT, a photosensitising porphyrin-based drug is systemically administered followed by illumination of the target tissue with visible light, leading to the generation of singlet oxygen and local cytotoxic agents. Cell death is achieved by direct cell apoptosis or indirectly through a local inflammatory response (23). The procedure is usually performed with a flexible bronchoscope under moderate sedation and topical anaesthesia. The extent of the tumour should be mapped and any synchronous airway lesions should be confirmed or excluded before the PDT procedure.
Centrally located early-stage endobronchial lesions <1 cm in thickness without cartilage or lymph node involvement can be treated efficiently by PDT. These lesions are occasionally occult or overt on conventional imaging modalities and are detected by screening sputum cytology in high-risk populations. Kato et al. (24) treated 264 stage 0 or I centrally located squamous-cell lung cancers and found that the CR rate was 95% for a maximum tumour length <5 mm, 94% in those 5–9 mm, and 44% in those >20 mm. Furuse et al. (25) reported similar findings: the CR rate was 100% in lesions <5 mm, whereas it was 38% for tumours >20 mm. Several series have reported that the CR rates for PDT range from 72% to over 90% for centrally located early-stage lung cancers (26-28).
Okunaka et al. (29) assessed the use of percutaneous PDT in nine patients with peripheral lung tumours <1 cm and seven achieved a partial response. An anecdotal study reported the use of endobronchial PDT in peripheral lung cancer with the help of a novel 1-mm-diameter composite-type optical fiberscope (30). More data are warranted to support the use of PDT for managing peripheral NSCLC in the future.
Complications following catheter-based treatment
The most common complication of percutaneous RFA, MWA, and CRA is a pneumothorax, which occurs in as high as 39–63% of the patients, and 8–38.9% of them require chest tube drainage (16,31). The incidence of pneumothorax is considered to be associated with the length of the procedure and the number of the lesions treated. Very rarely, bronchopleural fistula can happen and lead to a persistent pneumothorax that fails to relieve with simple chest draining. Other infrequent complications include pneumonia, pleural effusion, pulmonary abscess and haemoptysis which happen in less than 3% of the treated patients.
By contrast, the primary adverse event of PDT is phototoxicity related to systemic administration of the photosensitisers and exposure to ultraviolet light (32). Such photosensitivity usually lasts for 4–6 weeks and patients are advised to avoid direct sunlight exposure. Additionally, it is suggested to have a repeat bronchoscopy 2 days following PDT to remove any tissue debris and retained secretions and to eliminate any bronchial obstruction.
Challenges when evaluating the treatment response
The ablation margin is usually determined by measuring the lucent area surrounding the tumour. As target volume increases, the periphery may not reach an ablative temperature, resulting in a diminished response and impaired local control. Local progression is generally defined as enhancement along the rim of the lesion on CT. Due to thermally induced inflammation, interpreting the tumour response within 3 months following the procedure is difficult (31). Thus, imaging follow-up is indispensable to evaluate local recurrence. The size and density of the lesion on CT, as well as the standardised uptake value on positron emission tomography should be integrated together for evaluation of the tumour response (33). It is also stressed that the striped pattern ablation zone that transforms into a nodular pattern should be strictly followed up because of its tendency for local progression (34).
Technical combination with hybrid thoracic theatre
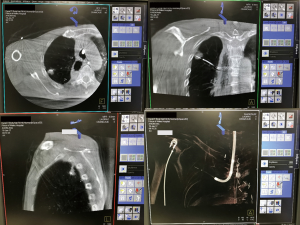
The integration of real-time imaging guidance technology in a hybrid theatre can assist precise localisation of a small pulmonary tumour (35). Therefore, the diagnostic and therapeutic procedures can be accomplished in a one-step workflow. Physicians and surgeons can easily adjust the electrode/probe for ablation under the guidance of cone-beam CT (Figure 1). Novel technology has allowed RFA to be conducted under guidance of CT-imaging bronchoscopy (36), which is believed to result in lower complication rates than transthoracic techniques. In a recent study (15), CT-guided bronchoscopy cooled RFA showed tumour control comparable to the percutaneous route in treating 23 peripheral stage I lung cancers. Interestingly, only three of the patients experienced fever and chest pain, which was managed conservatively, and no severe complications occurred. It is plausible that more advanced bronchoscopy, such as electromagnetic navigation bronchoscopy (37), as well as the development of micro-robotic platforms could enable more peripherally located lesions to be accurately reached for ablation treatment within the hybrid suite setting (38).
Conclusion and future directions
In summary, patients with early-stage NSCLC deemed high-risk, medically inoperable or otherwise unsuitable for SBRT have the treatment options described above. Emerging evidence shows that such catheter-based therapies are feasible and safe, with manageable morbidities and toxicities. Evaluation of the treatment response can be challenging, particularly in the early post-ablation period. Biopsy should be considered when there is suspicion of a relapse. The generalisability of these survival data is limited, and larger prospective studies with long-term follow-ups are needed to elucidate the role of catheter-based therapies in the future.
In addition, the advent of the hybrid thoracic theatre integrating advanced instrument allows precise localization of the target lesion and delivery of interventional therapy. Periphery lesions that traditionally require percutaneous access are expected to be approached under navigational endobronchial route, which could minimize the treatment-associated complications. It is possible that a future scenario for clinical early-stage lung cancer patients with a borderline pulmonary function would be to perform the diagnostic and therapeutic procedures in the same setting in the hybrid suite. However, it should be noted that the alternative treatments described in this narrative review should always be selected carefully after assessing the tumour’s biology and molecular alternations by a multi-disciplinary team.
Acknowledgements
None.
Footnote
Conflicts of Interest: CS Ng has an electromagnetic navigational bronchoscopy system SuperDimension Version 7 on loan from Medtronic. ZR Zhao and RW Lau have no conflicts of interest to declare.
References
- Ng CS, Zhao ZR, Lau RW. Tailored Therapy for Stage I Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:268-70. [Crossref] [PubMed]
- Garzon JC, Ng CS, Sihoe AD, et al. Video-Assisted Thoracic Surgery Pulmonary Resection for Lung Cancer in Patients with Poor Lung Function. Ann Thorac Surg 2006;81:1996-2003. [Crossref] [PubMed]
- Garzon JC, Ng CS, Lee TW, Yim AP. Video-Assisted Thoracic Surgery Lung Resection Following Endobronchial Valves Placement. J Thorac Cardiovasc Surg 2006;131:499-500. [Crossref] [PubMed]
- Zhao ZR, Situ DR, Lau RW, et al. Comparison of Segmentectomy and Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol 2017;12:890-6. [Crossref] [PubMed]
- El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 815-6. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Abbas G, Pennathur A, Landreneau RJ, et al. Radiofrequency and microwave ablation of lung tumors. J Surg Oncol 2009;100:645-50. [Crossref] [PubMed]
- Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol 2008;15:1765-74. [Crossref] [PubMed]
- Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:160-6. [Crossref] [PubMed]
- Hiraki T, Gobara H, Iishi T, et al. Percutaneous radiofrequency ablation for clinical stage I non-small cell lung cancer: results in 20 nonsurgical candidates. J Thorac Cardiovasc Surg 2007;134:1306-12. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 2007;243:268-75. [Crossref] [PubMed]
- Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg 2007;134:857-64. [Crossref] [PubMed]
- Ambrogi MC, Fanucchi O, Cioni R, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol 2011;6:2044-51. [Crossref] [PubMed]
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. [Crossref] [PubMed]
- Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration 2015;90:47-55. [Crossref] [PubMed]
- Brace CL, Hinshaw JL, Laeseke PF, et al. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology 2009;251:705-11. [Crossref] [PubMed]
- Jones GC, Kehrer JD, Kahn J, et al. Primary Treatment Options for High-Risk/Medically Inoperable Early Stage NSCLC Patients. Clin Lung Cancer 2015;16:413-30. [Crossref] [PubMed]
- Han X, Yang X, Ye X, et al. Computed tomography-guided percutaneous microwave ablation of patients 75 years of age and older with early-stage nonsmall cell lung cancer. Indian J Cancer 2015;52 Suppl 2:e56-60. [Crossref] [PubMed]
- Acksteiner C, Steinke K. Percutaneous microwave ablation for early-stage non-small cell lung cancer (NSCLC) in the elderly: a promising outlook. J Med Imaging Radiat Oncol 2015;59:82-90. [Crossref] [PubMed]
- Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998;37:171-86. [Crossref] [PubMed]
- Yamauchi Y, Izumi Y, Hashimoto K, et al. Percutaneous cryoablation for the treatment of medically inoperable stage I non-small cell lung cancer. PLoS One 2012;7:e33223. [Crossref] [PubMed]
- Moore W, Talati R, Bhattacharji P, et al. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol 2015;26:312-9. [Crossref] [PubMed]
- Simone CB 2nd, Friedberg JS, Glatstein E, et al. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis 2012;4:63-75. [PubMed]
- Kato H, Usuda J, Okunaka T, et al. Basic and clinical research on photodynamic therapy at Tokyo Medical University Hospital. Lasers Surg Med 2006;38:371-5. [Crossref] [PubMed]
- Furuse K, Fukuoka M, Kato H, et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J Clin Oncol 1993;11:1852-7. [Crossref] [PubMed]
- Corti L, Toniolo L, Boso C, et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 2007;39:394-402. [Crossref] [PubMed]
- Usuda J, Ichinose S, Ishizumi T, et al. Management of multiple primary lung cancer in patients with centrally located early cancer lesions. J Thorac Oncol 2010;5:62-8. [Crossref] [PubMed]
- Endo C, Miyamoto A, Sakurada A, et al. Results of long-term follow-up of photodynamic therapy for roentgenographically occult bronchogenic squamous cell carcinoma. Chest 2009;136:369-75. [Crossref] [PubMed]
- Okunaka T, Kato H, Tsutsui H, et al. Photodynamic therapy for peripheral lung cancer. Lung Cancer 2004;43:77-82. [Crossref] [PubMed]
- Usuda J. Photodynamic Therapy for Peripheral Lung Cancers Using Composite-Type Optical Fiberscope of 1.0 mm in Diameter. Am J Respir Crit Care Med 2017;195:A7637.
- Klapper JA, Hittinger SA, Denlinger CE. Alternatives to Lobectomy for High-Risk Patients With Early-Stage Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;103:1330-9. [Crossref] [PubMed]
- Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J 2006;28:200-18. [Crossref] [PubMed]
- Herrera LJ, Fernando HC, Perry Y, et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg 2003;125:929-37. [Crossref] [PubMed]
- Inoue M, Nakatsuka S, Jinzaki M. Cryoablation of early-stage primary lung cancer. Biomed Res Int 2014;2014:521691. [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid Theater and Uniportal Video-Assisted Thoracic Surgery: The Perfect Match for Lung Nodule Localization. Thorac Surg Clin 2017;27:347-55. [Crossref] [PubMed]
- Tanabe T, Koizumi T, Tsushima K, et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest 2010;137:890-7. [Crossref] [PubMed]
- Ng CS, Yu SC, Lau RW, et al. Hybrid Dyna- CT Guided Electromagnetic Navigational Bronchoscopic Biopsy. Eur J Cardiothorac Surg 2016;49:i87-8. [PubMed]
- Ng CS, He JX, Rocco G. Innovations and technologies in thoracic surgery. Eur J Cardiothorac Surg 2017;52:203-5. [Crossref] [PubMed]


