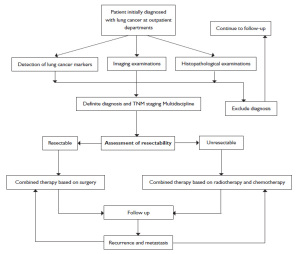
Treatment principles
A comprehensive treatment plan should be developed, i.e. to achieve radical cure or maximize the control of tumor and improve the cure rate, improve the patient's quality of life and prolong survival by a well-planned multidisciplinary treatment model (MDT) combining surgery, chemotherapy, radiotherapy and targeted biological therapy based on his/her conditions, tumor cytology, pathological type, extension (clinical stage) and development trends. Currently, the treatment of lung cancer is still built on surgery, radiotherapy and medication.
Surgery
Principles
Surgical resection is the mainstream treatment of lung cancer and the only way to achieve clinical cure. Common approaches include radical surgery and palliative surgery, where radical resection is always the ultimate goal as it may completely remove tumors, reduce tumor metastasis and recurrence, and conduct the final pathological TNM staging to inform postoperative comprehensive treatment. The following surgical principles must be observed for resectable lung cancer:
- A comprehensive treatment plan and necessary imaging studies (clinical staging and examination) must be completed before non-emergency surgery. Surgical protocols should be based on a full assessment of the possibility of surgical resection;
- Attempts should be made to completely remove tumors and regional lymph nodes while retaining functional lung tissues;
- Video-assisted thoracoscopic surgery (VATS) is a rapidly developing minimally invasive surgical technique in recent years, particularly applicable to patients with stage I lung cancer;
- Should the patient's physical condition allows, anatomic pulmonary resection (lobectomy, bronchial sleeve lobectomy or pneumonectomy) must be performed. Otherwise, limited resection -- pulmonary resection (preferred) or wedge resection -- or VATS may be chosen;
- If complete resection (R0 surgery) is performed, hilar and mediastinal lymph nodes (N1 and N2 lymph nodes) must be removed, located and sent for pathology in addition to complete removal of the primary lesions. Sampling or dissection must be done at least for three mediastinal drainage areas (N2 groups) by making the best use of en bloc resection of lymph nodes. Preferably, dissection should include 2R, 3a, 3p, 4R and 7-9 groups of lymph nodes and the surrounding soft tissues of the right chest, and 4L and 5-9 groups of lymph nodes and the surrounding soft tissues of the left chest;
- The surgery should in turn involve the pulmonary vein, pulmonary artery, and finally the bronchi;
- The lung function (including bronchi or pulmonary artery) should be preserved as much as possible given that negative margins (including bronchial, pulmonary artery or vein ends) are confirmed by rapid intraoperative pathological examination in sleeve lobectomy. This may result in better quality of life after surgery compared with patients undergoing complete resection;
- If recurrence or solitary pulmonary metastasis is present 6 months after complete resection of lung cancer, resection of the ipsilateral residual lung or metastatic lesions could be the choice as long as distant metastasis is excluded; and
- Surgery-ineligible patients with stages I and II diseases due to cardiovascular conditions or other findings may receive radical radiotherapy, radiofrequency ablation and drug therapy as alternatives.
Indications
- Stages I, II, III and some IIIa (T3N1-2M0, T1-2N2M0 and T4N0-1M0 can be completely resected) non-small cell lung cancer, and some small cell lung cancer (T1-2N0-1M0);
- Stage N2 non-small cell lung cancer responsive to neoadjuvant treatment (chemotherapy or chemotherapy plus radiotherapy);
- Some stage IIIb non-small cell lung cancer (T4N0-1M0) if complete resection of localized lesion is possible, including those involving the superior vena cava or adjacent to large blood vessels, heart, carina and so on;
- Some stage IV non-small cell lung cancer with a single contralateral lung metastasis, single brain or adrenal metastasis; or
- Surgical exploration is made when a definite diagnosis is not possible for an intrapulmonary nodule highly suspected of lung cancer.
Contraindications
Surgery ineligibility or intolerability due to systemic conditions or poor functions of the heart, lung, liver, kidney and other vital organs; or
Most of the confirmed stage IV, a majority of stage IIIb and some stageIIIa non-small cell lung cancer, as well as small cell lung cancer beyond the T1-2N0-1M0 stage.
Radiation therapy
Radiation therapy for lung cancer includes radical radiotherapy, palliative radiotherapy, adjuvant radiotherapy and prophylactic radiotherapy.
Principles
- Radical radiotherapy is applicable to patients with a KPS score ≥ 70 points (refer to Karnofsky score in Table 2), including those with inoperable early-stage non-small cell lung cancer, unresectable locally advanced non-small cell lung cancer and limited-stage small cell lung cancer due to iatrogenic and/or personal factors;
- Palliative radiotherapy is used to reduce symptoms associated with primary lesions and metastases of advanced lung cancer. Whole-brain radiotherapy can be performed in patients who have a single brain metastasis of non-small cell lung cancer that is surgically removed;
- Adjuvant radiotherapy is suitable for patients who have received preoperative radiotherapy and have positive surgical margins. Postoperative pN2-positive patients are encouraged to participate in clinical research;
- The postoperative radiotherapy protocol should be built on surgical pathology reports and surgical records;
- Prophylactic radiotherapy is used as a whole-brain approach for patients with small cell lung cancer responsive to systemic treatment;
- Radiotherapy is usually performed in combination with chemotherapy for lung cancer. In view of different stages, treatment objectives and patients' general conditions, it may be combined with concurrent chemotherapy or sequential chemotherapy. Concurrent radio-chemotherapy uses EP and paclitaxel-containing treatment regimens;
- Patients should be informed before treatment of increased potential toxic side effects as a result of chemotherapy. Radiotherapy should be designed and implemented in such a way that the lungs, heart, esophagus and spinal cord are well protected. The risk of unplanned interruption due to improper treatment of toxic side effects should be minimized during radiotherapy;
- The application of other advanced techniques, such as three-dimensional conformal radiotherapy (3DCRT) and intensity modulated radiotherapy (IMRT), are recommended; and
- Adequate monitoring and supportive care should be provided to patients undergoing radiotherapy or chemotherapy during the rest period.
Indications of radiotherapy for non-small cell lung cancer (NSCLC)
Radiotherapy can be used as a radical treatment for early-stage NSCLC that is inoperable due to poor health status, or as a pre- and post-operative adjuvant therapy for operable lesions, local treatment for advanced, unresectable local lesions, and an important palliative approach for advanced incurable patients.
Radiotherapy is an effective way of local lesion control for surgery-ineligible patients with stage I NSCLC. For NSCLS patients that have negative surgical margins but positive mediastinal lymph nodes (pN2), the addition of posteropative radiotherapy is recommended to routine adjuvant chemotherapy. Postoperative concurrent radio-chemotherapy is recommended, if tolerable, for pN2 tumors with a positive margin. Radiotherapy should be initiated as soon as possible for margin-positive patients.
For patients with stages II to III NSCLC who are ineligible for surgery due to poor health status, concurrent radio-chemotherapy may be administered if tolerable. A more suitable radiotherapy plan and more aggressive supportive care may be provided to those in whom cure is possible during their radiotherapy or chemoradiotherapy, so as to minimize treatment interruption and dose reduction.
Some patients with widespread metastatic stage IV NSCLC may accept palliative radiotherapy for both the primary tumor and metastases in order to reduce symptoms.
Indications of radiotherapy for small cell lung cancer (SCLC)
Complete remission is possible for some limited-stage SCLC patients through systemic chemotherapy. However, there is a high risk of intrathoracic recurrence if chest radiotherapy is not added. This addition may significantly reduce not only the local recurrence rate but also the risk of death.
In patients with extensive-stage SCLC, the addition of chest radiotherapy after chemotherapy of distant metastases also improves the tumor control rate and prolongs survival.
If the patient's conditions permit, radiotherapy for small cell lung cancer should be started as soon as possible -- simultaneous administration with chemotherapy may be considered. For large lesions where the lungs are at risk of being damaged by radiotherapy, 2-3 cycles of chemotherapy can be administered followed by radiotherapy as soon as possible.
Prophylactic cranial radiotherapy
For patients with limited-stage small cell lung cancer, prophylactic cranial radiotherapy is recommended following treatment if complete remission of intrathoracic lesions has been achieved. Prophylactic cranial radiotherapy may also reduce the risk of brain metastasis in patients with extensive-stage small cell lung cancer that is responsive to chemotherapy.
Decisions to start prophylactic whole-brain radiotherapy for patients with non-small cell lung cancer should be based on full communication between doctors and patients and the goal to balance individual benefits and risks.
Palliative radiotherapy for patients with advanced lung cancer
Palliative radiotherapy is provided for patients with advanced lung cancer to relieve local compression symptoms cause by primary tumors or metastases, pain caused by bone metastases, and neurological symptoms due to brain metastases. Low-dose fractionated radiotherapy may be considered in such patients for ease of treatment and rapid relief of symptoms.
Efficacy
Efficacy is assessed according to the WHO Response Evaluation Criteria in Solid Tumors (
Table 3) or RECIST Response Evaluation Criteria (
Table 4).
Protection
The lungs, heart, esophagus and spinal cord must be well protected when using conventional radiotherapy techniques to avoid serious radiological damage to vital organs. Acute radiation-induced lung injury is assessed according to the RTOG standards (
Table 5).
Drug therapy for lung cancer
Drug therapy for lung cancer includes chemotherapy and molecular targeted therapy (EGFR-TKI therapy). Chemotherapy consists of palliative chemotherapy, adjuvant chemotherapy and neoadjuvant chemotherapy, which should be administered on strict indications and under the guidance of oncologists. The stages of disease, physical condition, adverse reactions, quality of life and patient's wishes should be taken into account when delivering chemotherapy to avoid over-treatment or inadequate treatment. Assessment of the chemotherapy efficacy should be conducted in a timely manner along with close monitoring and control of adverse reactions, so that drugs and/or doses could be adjusted accordingly.
Indications for chemotherapy: PS score ≤ 2 (5-point-scale ZPS score,
Table 6), function reserve of vital organs enable the patient to tolerable chemotherapy; SCLC patients with a PS score up to 3 are also eligible. Patients are encouraged to participate in clinical trials.
Drug therapy for advanced NSCLC
- First-line regimens: A platinum-based two-drug combination is the standard first-line treatment; patients with EGFR mutations may choose targeted therapy drugs. Anti-angiogenesis drugs may also be added when applicable. Currently available chemotherapy drugs are listed in Table 7. Patients who have achieved disease control (CR + PR + SD) by first-line therapy may choose maintenance therapy if conditions permit.
- Second-line regimens: The second-line therapy options include docetaxel, pemetrexed and targeted drug EGFR-TKI; and
- Third-line regimens EGFR-TKI may be used. Otherwise, patients may also be encouraged to participate in a clinical trial.
Drug therapy for unresectable NSCLC
A combination of radiotherapy and chemotherapy is recommended. The use of concurrent or sequential radio-chemotherapy may depend on the particular case. Etoposide/cisplatin or carboplatin (EP/EC) and paclitaxel or docetaxel/cisplatin are recommended for concurrent regimens. First-line therapy drugs can be used in sequential chemotherapy.
T2b: Tumor more than 5 cm but 7 cm or less in greatest dimension.
Perioperative adjuvant therapy for NSCLC
Three or four cycles of platinum-containing two-drug adjuvant chemotherapy are recommended after completely removal of stage II-III NSCLC. Postoperative adjuvant chemotherapy often begins 3-4 weeks after surgery when the patient's physical conditions have returned to normal.
Neoadjuvant chemotherapy: two cycles of preoperative neoadjuvant chemotherapy with platinum-containing two-drug regimens may be considered for resectable stage III NSCLC. Timely assessment of the efficacy and monitoring of adverse reactions are needed to avoid surgical complications. Surgery is generally performed 2-4 weeks after the chemotherapy. Postoperative adjuvant therapy regimens are dependent on the preoperative staging and efficacy of neoadjuvant chemotherapy; maintenance or adjustment of the original protocol for tolerability considerations may be possible. The regimen may also be replaced if ineffective.
Drug therapy for small cell lung cancer (SCLC)
Radiotherapy and chemotherapy-based comprehensive treatment is recommended for limited-stage small cell lung cancer (stage II-III). EP or EC is recommended as chemotherapy regimens.
Chemotherapy-based comprehensive treatment is recommended for extensive-stage small cell lung cancer (stage IV). EP, EC or cisplatin plus topotecan (IP) or irinotecan (IC) are recommended as the chemotherapy drugs.
Topotecan is a recommended second-line option. Patients are encouraged to participate in new drug clinical studies.
Principles of cancer chemotherapy
- Patients with KPS 2 are not candidates for chemotherapy;
- Patients whose white blood cell count is less than 3.0×109/L, neutrophil count less than 1.5×109/L, platelet count less than 6×109/L, red blood cell count less than 2×109/L and hemoglobin less than 8.0 g/dl are not candidates for chemotherapy in principle;
- Patients with liver and kidney dysfunction, abnormal laboratory indicators (higher than 2 times normal), or those who have serious complications and infections, fever or bleeding predisposition are not candidates for chemotherapy;
- Discontinuation or change of chemotherapy regimens should be considered if the following occurs: Disease progression after 2 cycles of therapy, or deterioration again during the rest period, which requires discontinuation of the original regimen and switch to others; grade 3/4, obviously life-threatening adverse reactions; or serious complications, which requires discontinuation of the current regimen and switch to another for the next treatment course; and
- Standardized and individualized chemotherapy protocols are key to successful treatment. The basic requirements of chemotherapy should be followed. In addition to routine antiemetic drugs, hydration and diuretics are required for platinum-based regimens except for carboplatin. Blood tests are performed twice a week after chemotherapy;
- Efficacy is assessed according to the WHO Response Evaluation Criteria in Solid Tumors.
Stage-based treatment for non-small cell lung cancer
Combined treatment for stage I non-small cell lung cancer
- Surgery is always the treatment of choice, including lobectomy plus hilar and mediastinal lymph node dissection; thoracotomy or VATS can be used;
- For patients with poor lung function, anatomic pulmonary segment or wedge resection plus hilar and mediastinal lymph node dissection may be considered;
- Patients undergoing complete resection of stage IA lung cancer are not candidates for postoperative adjuvant chemotherapy;
- Routine use of adjuvant chemotherapy not recommended in patients with completely resected stage IB lesions;
- Re-operation is recommended for stage I patients with positive surgical margins. Postoperative chemotherapy and radiotherapy is preferred for patients not suitable for reoperation, regardless of reasons.
Combined treatment for stage II non-small cell lung cancer
- Surgical treatment is preferred, including lobectomy, double lobectomy or pneumonectomy plus hilar and mediastinal lymph node dissection;
- For patients with poor lung function, anatomic pulmonary segment or wedge resection plus hilar and mediastinal lymph node dissection may be considered;
- Postoperative adjuvant chemotherapy is recommended for those undergoing complete resection of stage 2 non-small cell lung cancer;
- En bloc resection should be performed if pleural or chest wall invasion is present. Resection of ribs, at least from recent lesions of the upper and lower edge 2cm, invaded rib resection should be at least when away from the tumor 5cm.
- Reoperation is recommended for stage II patients with positive surgical margins. Postoperative chemotherapy plus radiotherapy is preferred for patients not suitable for reoperation, regardless of reasons.
Combined treatment for stage III non-small cell lung cancer
Locally advanced non-small cell lung cancer refers to stage III lung cancer according to the TNM staging system. Comprehensive treatment is the best option for stage III non-small cell lung cancer. There are two types of locally advanced NSCLC: resectable and unresectable, where:
1. Resectable locally advanced non-small cell lung cancer includes:
T3N1 NSCLC, for which surgical treatment is prefered followed by postoperative adjuvant chemotherapy;
N2 lung cancer, for which surgery is controversial. Preoperative mediastinoscopy is recommended for those with a single set of enlarged mediastinal lymph nodes, or two sets of enlarged mediastinal lymph nodes without fusion and complete resection is expected, as revealed by imaging approaches. Preoperative neoadjuvant chemotherapy followed by surgical treatment may be performed upon definite diagnosis;
For some T4N0-1 tumors: a) satellite nodules within a single lobe: these are stage T3 as per the new staging system and the treatment of choice is surgical resection; preoperative neoadjuvant chemotherapy and postoperative adjuvant chemotherapy can also be considered; b) for other resectable T4N0-1 non-small cell lung cancer, neoadjuvant chemotherapy or surgical resection may be preferred. Adjuvant chemotherapy may be considered after complete resection. In the case of positive margins, postoperative radiotherapy and platinum-based chemotherapy are justified.
Treatment for Pancoast tumors: concurrent radio-chemotherapy followed by surgery plus adjuvant chemotherapy is recommended for some of the operable patients. For inoperable Pancoast tumor, radiotherapy and chemotherapy should be performed;
2. Unresectable locally advanced non-small cell lung cancer includes:
mediastinoscopy-positive non-small cell lung cancer with radiologically indicated massive mediastinal shadow;
most of the T4 and N3 non-small cell lung cancer;
T4N2-3 patients;
patients with pleural metastases, malignant pleural effusion and malignant pericardial effusion have been classified as M1 as per the new staging system, and are not candidates for surgery. Some cases may receive thoracoscopic pleural biopsy or pleurodesis.
Treatment for stage IV non-small cell lung cancer
Before treatment is initiated, a testing may be necessary to identify mutations in the epidermal growth factor receptor (EGFR). Subsequent treatment strategies may be developed in view of the EGFR mutation status.
Systemic treatment is the main approach to managing stage IV lung cancer, as the treatment would aim to improve the patient's quality of life and prolong survival.
1. Treatment for solitary metastasis of stage IV lung cancer
For solitary brain metastasis from resectable non-small cell lung cancer, the brain lesion can be resected or subject to stereotactic radiotherapy, while the primary lesion is treated according to the stage-based treatment schemes;
For solitary adrenal metastasis from resectable non-small cell lung cancer, the adrenal lesions may be resected while the primary lesion is treated according to the stage-based treatment schemes;
Stage-specific treatment is conducted for other solitary nodules in the ipsilateral or contralateral lung.
2. Systemic treatment for stage IV lung cancer
Gefitinib or erlotinib first-line treatment is recommended for stage IV non-small cell lung cancer harboring sensitive EGFR mutations;
For stage IV non-small cell lung cancer with wild-type or unknown mutant EGFR status and a functional status score PS between 0 and 1, platinum-containing two-drug chemotherapy should be initiated as soon as possible. Non-platinum-based two-drug regimens may be considered for those ineligible for platinum-containing chemotherapy;
Cytotoxic single-drug chemotherapy is used for advanced non-small cell lung cancer with PS=2, though there is no evidence that it is suitable for patients with PS>2;
Current evidence does not support age as a basis for chemotherapy selection;
Docetaxel and pemetrexed second-line chemotherapy, as well as gefitinib or erlotinib second- or third-line oral treatment, are recommended following failure of first-line chemotherapy for non-small cell lung cancer;
Optimal supportive care alone can be considered for patients with stage IV non-small cell lung cancer whose PS is higher than 2.
In addition to systemic treatment, proper local treatment may be used based on specific local conditions in order to improve symptoms and the patient's quality of life.
Stage-based treatment for small cell lung cancer
Stage I SCLC. Surgery plus adjuvant chemotherapy (EP/EC, 4-6 cycles).
Stage II-III SCLC: radiotherapy plus chemotherapy.
- Sequential or concurrent protocols may be selected;
- Recommended sequential therapy includes 2 cycles of induction chemotherapy followed by concurrent radio-chemotherapy;
- Prophylactic cranial irradiation (PCI) is recommended for patients who have achieved disease control after standard treatment.
Stage IV SCLC: Chemotherapy-based comprehensive therapy with a view to improve the quality of life.
Recommended first-line drugs include EP/EC, IP and IC. Patients with recurrence or progression within 3 months of standard treatment are encouraged to participate in clinical trials. Topotecan, irinotecan, gemcitabine or paclitaxel may be considered for patients with recurrence within 3-6 months. The initial treatment program may be used for those having disease progression beyond 6 months.