Left ventricular diastolic dysfunction in patients with obesity hypoventilation syndrome
Introduction
Obesity is a serious health concern worldwide (1). It is associated with an increased risk of cardiovascular diseases, diabetes, chronic pain, asthma, and obesity hypoventilation syndrome (OHS) (2,3). The exact pathophysiological mechanisms responsible for OHS have yet to be clearly defined. Proposed theories include complex interactions among impaired respiratory mechanics, abnormal central ventilatory control, possible sleep-disordered breathing, and neurohormonal aberrancies (4).
OHS patients have a progressive disease course, high health care utilization, and higher risks of hospitalization and death (5,6). The associated respiratory, metabolic, hormonal, and cardiovascular impairments in OHS can lead to lower quality of life and increased morbidity and mortality (7). Only a few studies have assessed cardiac complications in patients with OHS, and these focused mainly on right ventricular dysfunction and pulmonary hypertension (PH) (8,9).
Several recent reports have suggested that left ventricular dysfunction in patients with OHS could be mainly due to diastolic dysfunction (10,11). However, these studies were retrospective and included a small sample size. We hypothesize that left ventricular diastolic dysfunction (LVDD) is common among patients with OHS. Therefore, we designed this study to more comprehensively assess the prevalence, clinical characteristics, and factors associated with LVDD in patients with OHS.
Methods
This study is a prospective observational study conducted between January 2002 and December 2016 at King Saud University Medical City, a tertiary-care University hospital in Riyadh, Saudi Arabia. The local ethics committee approved the study, and informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki. We included all consecutive patients (≥18 years old) who met the diagnostic criteria of OHS, body mass index (BMI) >30 kg/m2, daytime awake PaCO2 >45 mmHg, and in whom hypoventilation was not primarily due to a chronic lung disease, chest wall deformity, medications, a neuromuscular disorder, or a known congenital or idiopathic central alveolar hypoventilation syndrome (12). None of the studied patients was in acute hypercapnic respiratory failure at the time of echocardiography. Hypertensive patients were defined as those who had a documented history of hypertension or were on antihypertensive treatment. Diabetes was defined as HbA1C >6.5 or being on antidiabetic agents. Exclusion criteria included patients with chronic liver or renal disease, recent myocardial infarction, atrial fibrillation, and moderate or severe valvular heart disease.
Demographic data including co-morbidities, smoking history, and BMI were recorded. Additionally, all patients underwent transthoracic echocardiography, pulmonary function test (PFT), and arterial blood gases (ABGs) after 15 minutes of rest while the subjects were awake, seated, and breathing room air. The blood gases were analyzed using a GEM® Premier™ 4000 analyzer (Instrumentation Laboratory, Lexington, MA, USA).
Polysomnography
Patients underwent a type-I attended overnight sleep study with neurological, cardiac, and respiratory monitoring using Alice® diagnostic equipment (Philips, Respironics Inc., Murrysville, PA, USA) (13,14). Scoring was performed manually according to the American Academy of Sleep Medicine (AASM) scoring criteria (15). The desaturation index was defined as the number of desaturation events (≥3% decrease in oxygen saturation from the pre-event baseline) per hour of sleep.
Echocardiography
Echocardiography was carried out using a Philips iE33 (Philips Ultrasound Bothell, WA, USA) cardiac ultrasound machine, with an electronic transducer of variable frequency and capacity for two-dimensional (2D), M-mode, continuous, and pulsed wave Doppler and color images. The examination was performed in M-mode with 2D guidance in the long axis of the left parasternal view. Left ventricular internal end-diastolic and end-systolic diameters, as well as interventricular septum and posterior wall thicknesses, were measured over five consecutive cycles (16). Systolic function was assessed by the left ventricular ejection fraction according to the Teicholz formula (17). Left ventricular diastolic function was evaluated by transmitral Doppler using the pulsed-Doppler technique with 2D guidance in the apical four-chamber view. Left ventricular diastolic function can be measured by flow parameters including the early (E) and late (A) diastolic filling velocities, the E/A ratio, and the E deceleration time (DT) from an apical four-chamber view using the pulsed-Doppler technique with 2D guidance. The transmitral E wave is related to the time course of active left ventricular relaxation, which produces a pressure gradient from the left atrium through the left ventricular inflow tract to the left ventricular apex (18,19). Therefore, the early left ventricular diastolic filling is affected by the interaction of left atrial compliance and the rate of ventricular relaxation. The peak E velocity may increase by minimal left ventricular diastolic pressure caused by rapid left ventricular relaxation, or elevated left atrial pressure (the cause of high E/A ratios in cardiac disease) (19,20). Impaired left ventricular relaxation denotes grade 1 diastolic dysfunction, grade 2 is pseudonormalization, and grades 3 and 4 are diagnosed when there is restrictive physiology (21). All echocardiographic tests were performed by the same experienced echocardiographer.
Statistical analysis
Continuous variables of the study population characteristics were expressed as the mean ± standard deviation or as the median (lower percentile, upper percentile) if normality assumption failed. Categorical data were expressed as n (%). Comparisons between two categorical variables were made using the chi-square test. Continuous data were compared using student t-test or Mann Whitney U-test depending on data distribution. A P value less than 0.05 was considered statistically significant. Pearson’s correlation coefficient was used to assess the association of study parameters with diastolic dysfunction. Statistical Package for Social Sciences software (SPSS version 22; IBM Corporation, Armonk, NY, USA) was used for data analysis and management.
Results
A total of 126 patients met the inclusion and exclusion criteria and agreed to participate. Eleven patients had moderately reduced ejection fraction (EF) (35–40%) and two had severe left ventricular systolic dysfunction (EF 25–30%). These patients (n=13) were excluded. Figure 1 shows the distribution of the recruited patients.
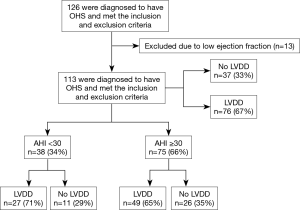
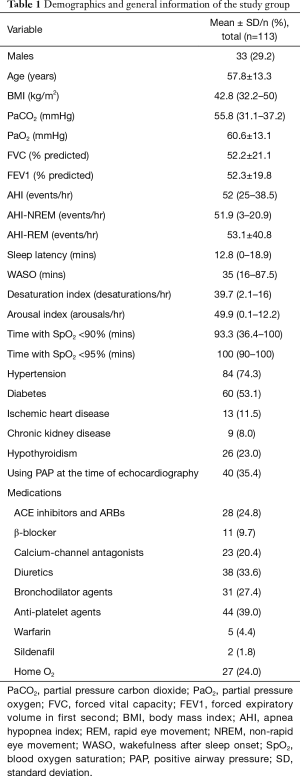
In the finally included group (n=113), the mean age of the participants was 57.8±13.3 yrs. The median BMI was 42.8±11.5 kg/m2. Males represented 29.2% (n=33). The median arterial partial pressure of carbon dioxide (PaCO2) was 55.68±13.35 mmHg. Approximately, 35% of patients were on positive airway pressure therapy at the time of cardiac evaluation, 84 (74.3%) patients were hypertensive and 60 (53.1%) were diabetic (Table 1).
Full table
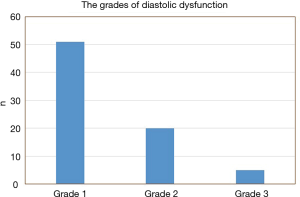
Of the 113 patients with OHS, 76 patients (67%) were found to have LVDD. Of those with LVDD, more than two-thirds (67%) had grade 1 diastolic dysfunction, and the rest had grades 2 and 3 (26% and 7%, respectively) (Figure 2).
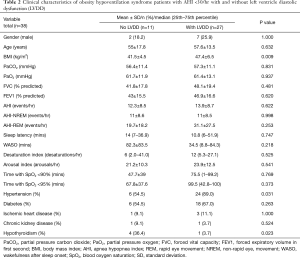
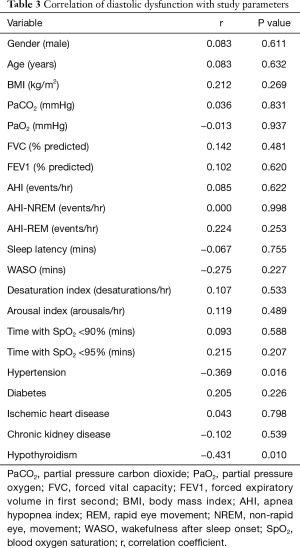
To minimize the effect of fluctuations in intrathoracic pressure during obstructive respiratory events on cardiac function, we did a subgroup analysis based on the apnea hypopnea index (AHI). Thirty-eight OHS patients with mild to moderate OSA (AHI <30) were identified (Table 2). Twenty-seven (71%) of them had LVDD; whereas 49 (65%) of patients with severe OSA (AHI ≥30) had LVDD (Figure 1). In the subgroup of OHS patients with mild to moderate OSA, OHS patients with LVDD had higher BMI compared to OHS patients without LVDD (47.4±6.5 kg/m2 versus 41.5±4.5 kg/m2, P=0.009). In addition, hypertension was more common in OHS patients with LVDD than without LVDD (89.0% versus 54.5%, P=0.03). There were no significant differences in age, partial pressure of oxygen or carbon dioxide, or in measured sleep parameters. Time spent with oxygen saturation SpO2 <90% and <95% was longer in the OHS with LVDD group; however, it did not reach statistical significance (Table 2). Correlation analysis revealed that hypertension (r=−0.37, P=0.016) significantly correlated with LVDD in OHS patients with mild OSA (Table 3).
Full table
Full table
Discussion
Although cardiac dysfunction is a known comorbidity of OHS, only a limited number of studies have addressed this topic. Our study is the largest prospective study to assess the prevalence of LVDD among OHS patients, and to study LVDD in patients with OHS and mild to moderate OSA. We observed that the prevalence of LVDD was 67% in patients with OHS, and 71% in a sub-group of OHS patients with AHI <30, which is much higher than the prevalence reported in the public ranging from 11–34% (22,23). The majority of LVDD in OHS patients were mild, grade 1. Our results are in agreement with the findings of Alawami et al., who retrospectively reviewed 47 patients with OHS and reported left ventricular systolic dysfunction in 25% and LVDD in 60% of patients (10).
In the present study, the median AHI of patients with OHS was 52 events/hr. LVDD and left ventricular remodeling have been frequently reported in patients with OSA, and left ventricular dysfunction correlates with the severity of OSA and AHI (24). The reported prevalence of diastolic dysfunction in OSA is approximately 23% (25,26). Large swings in intrathoracic pressure in OSA patients may increase sympathetic activity and surges in blood pressure (27). Recurrent forced inspiration against the occluded airway during apnea episodes results in excessive negative intrathoracic pressure. Additionally, these pressure swings lead to an increased venous return to the right ventricle with an overload of the right ventricle, which pushes the interventricular septum to shift to the left, resulting in reduced left ventricular end-diastolic volume (28). In addition to increased blood pressure, these factors collectively cause diastolic dysfunction (29,30). In order to minimize the effect of fluctuations in intrathoracic pressure during the obstructive respiratory events on the cardiac function, we opted to analyze the subgroup of OHS patients with AHI <30 (31).
The prevalence of LVDD in OHS patients with mild to moderate OSA was high (71%), indicating that other factors, apart from repetitive obstructive events, contribute to the development of LVDD. Morbid obesity is a well-known risk factor for heart failure, even in the absence of other cardiovascular risk factors such as ischemic heart disease, hypertension, and diabetes (32,33). The incidence of obesity was reported to be 41.4% in patients discharged with a diagnosis of heart failure with preserved ejection fraction (34). Additionally, various neurohormonal and metabolic abnormalities associated with obesity, such as hyperinsulinemia, hyperleptinemia, as well as activation of the renin-aldosterone-angiotensin system and the sympathetic nervous system are thought to be responsible for cardiac remodeling, left ventricular hypertrophy and dysfunction, and altered left ventricular morphology (35). It is postulated that the inflammatory state and oxidative stress that develop with morbid obesity can lead to an increase in left ventricular mass including left ventricular internal diastolic chamber size and left ventricular wall thickness. Left ventricular mass correlates with BMI and mortality (21,35). In this study, there was a significant difference in BMI between OHS subjects with and without LVDD when severe OSA was excluded (Table 2).
Furthermore, sleep disordered breathing in itself can increase the risk of cardiovascular disease. A strong association with cardiovascular disease was found among obese patients with sleep apnea compared to matched controls of obese patients without sleep apnea (10,32,33). The chronic sustained hypoxemia that occurs in OHS causes sympathetic activation, systemic inflammation, oxidative stress, and metabolic abnormalities (31). These in turn result in left ventricular remodeling and left ventricular hypertrophy, which are the cardinal feature of diastolic dysfunction (36,37). On the other hand, hypercapnia exerts direct effects on cardiac myocytes due to an increase in acidity that results in depressed cardiac contractility and inhibition of glycolysis and the Krebs cycle (38,39).
Hypertension was more common in patients with LVDD than those without LVDD (Table 2). The chronic hypoxemia that occurs in OHS patients can cause a reflex elevation of the arterial blood pressure. This in turn may increase the work load of the heart and lead to left ventricular hypertrophy (38). Hypertension is a known risk factor for LVDD. Hypertension was diagnosed in 55–86% of a cohort of 2,843 patients with heart failure with preserved ejection fraction (39,40). Furthermore, respiratory acidosis that results from hypercapnia stimulates the sympathetic nervous system. The resultant tachycardia can further impair left ventricular filling and worsen LVDD (38).
Limitations of this study include the absence of a matched control group of obese patients; however, it is difficult to find middle-age morbidly obese patients without sleep disordered breathing. Another limitation is the fact that echocardiographic assessment of LVDD does not have very high specificity and positive predictive value, and the specificity may be compromised by conditions such as atrial fibrillation and mitral valve disease, which commonly cause left atrial dilatation (19,41). Future studies should use more advanced methods for cardiac assessments, such as cardiac MRI and left ventricular mass measurements (42).
In conclusion, LVDD is common among patients with OHS even without significant OSA. The identification of risk factors for LVDD may provide further insight into possible mechanisms underlying cardiac complications in this population. This will facilitate early recognition and provision of treatment. Targeting these risk factors may thus help to attenuate the development of LVDD.
Acknowledgements
Funding: This work was supported by the Strategic Technologies Program of the National Plan for Sciences and Technology and Innovation in the Kingdom of Saudi Arabia (MED511-02-08).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the institutional review board at King Saud University (ethics approval number is 11/3235/IRB), and informed consent was obtained from all of the participants prior to inclusion in this study.
References
- Ortega FB, Lavie CJ, Blair SN. Obesity and Cardiovascular Disease. Circ Res 2016;118:1752-70. [Crossref] [PubMed]
- Bastien M, Poirier P, Lemieux I, et al. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014;56:369-81. [Crossref] [PubMed]
- Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol 2005;115:897-909. [Crossref] [PubMed]
- Sequeira TCA. BaHammam AS, Esquinas AM. Noninvasive Ventilation in the Critically Ill Patient With Obesity Hypoventilation Syndrome: A Review. J Intensive Care Med 2017;32:421-8. [Crossref] [PubMed]
- Bahammam AS, Al-Jawder SE. Managing acute respiratory decompensation in the morbidly obese. Respirology 2012;17:759-71. [Crossref] [PubMed]
- Berg G, Delaive K, Manfreda J, et al. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001;120:377-83. [Crossref] [PubMed]
- Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med 2011;183:292-8. [Crossref] [PubMed]
- Almeneessier AS, Nashwan SZ, Al-Shamiri MQ, et al. The prevalence of pulmonary hypertension in patients with obesity hypoventilation syndrome: a prospective observational study. J Thorac Dis 2017;9:779-88. [Crossref] [PubMed]
- Kauppert CA, Dvorak I, Kollert F, et al. Pulmonary hypertension in obesity-hypoventilation syndrome. Respir Med 2013;107:2061-70. [Crossref] [PubMed]
- Alawami M, Mustafa A, Whyte K, et al. Echocardiographic and electrocardiographic findings in patients with obesity hypoventilation syndrome. Intern Med J 2015;45:68-73. [Crossref] [PubMed]
- Marik PE, Desai H. Characteristics of patients with the "malignant obesity hypoventilation syndrome" admitted to an ICU. J Intensive Care Med 2013;28:124-30. [Crossref] [PubMed]
- AASM. American Academy of Sleep Medicine (AASM). International classification of sleep disorders (ICSD), 3rd ed. Darien, IL: AASM. 2014.
- BaHammam AS. Prevalence, clinical characteristics, and predictors of obesity hypoventilation syndrome in a large sample of Saudi patients with obstructive sleep apnea. Saudi Med J 2015;36:181-9. [Crossref] [PubMed]
- BaHammam AS, Pandi-Perumal SR, Piper A, et al. Gender differences in patients with obesity hypoventilation syndrome. J Sleep Res 2016;25:445-53. [Crossref] [PubMed]
- Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.1. Darien, Illinois: American Academy of Sleep Medicine, 2014.
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63. [Crossref] [PubMed]
- Teichholz LE, Kreulen T, Herman MV, et al. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 1976;37:7-11. [Crossref] [PubMed]
- Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 1988;12:426-40. [Crossref] [PubMed]
- Mottram PM, Marwick TH. Assessment of diastolic function: what the general cardiologist needs to know. Heart 2005;91:681-95. [Crossref] [PubMed]
- Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol 1997;30:8-18. [Crossref] [PubMed]
- Dugo C, Rigolli M, Rossi A, et al. Assessment and impact of diastolic function by echocardiography in elderly patients. J Geriatr Cardiol 2016;13:252-60. [PubMed]
- Kloch-Badelek M, Kuznetsova T, Sakiewicz W, et al. Prevalence of left ventricular diastolic dysfunction in European populations based on cross-validated diagnostic thresholds. Cardiovasc Ultrasound 2012;10:10. [Crossref] [PubMed]
- Kuznetsova T, Herbots L, Lopez B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail 2009;2:105-12. [Crossref] [PubMed]
- Baguet JP, Barone-Rochette G, Tamisier R, et al. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol 2012;9:679-88. [Crossref] [PubMed]
- Bodez D, Damy T, Soulat-Dufour L, et al. Consequences of obstructive sleep apnoea syndrome on left ventricular geometry and diastolic function. Arch Cardiovasc Dis 2016;109:494-503. [Crossref] [PubMed]
- Chami HA, Resnick HE, Quan SF, et al. Association of incident cardiovascular disease with progression of sleep-disordered breathing. Circulation 2011;123:1280-6. [Crossref] [PubMed]
- Carlson JT, Hedner J, Elam M, et al. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 1993;103:1763-8. [Crossref] [PubMed]
- Shiomi T, Guilleminault C, Stoohs R, et al. Leftward shift of the interventricular septum and pulsus paradoxus in obstructive sleep apnea syndrome. Chest 1991;100:894-902. [Crossref] [PubMed]
- Arias MA, Garcia-Rio F, Alonso-Fernandez A, et al. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation 2005;112:375-83. [Crossref] [PubMed]
- Turgut Celen Y, Peker Y. Cardiovascular consequences of sleep apnea: II-Cardiovascular mechanisms. Anadolu Kardiyol Derg 2010;10:168-75. [Crossref] [PubMed]
- Wang J, Zhang H, Wu C, et al. Correlation of Left Ventricular Diastolic Function and Left Ventricular Geometry in Patients with Obstructive Sleep Apnoea Syndrome. West Indian Med J 2015;64:92-8. [PubMed]
- Alpert MA, Lavie CJ, Agrawal H, et al. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl Res 2014;164:345-56. [Crossref] [PubMed]
- Lavie CJ, Milani RV, Ventura HO, et al. Body composition and heart failure prevalence and prognosis: getting to the fat of the matter in the "obesity paradox". Mayo Clin Proc 2010;85:605-8. [Crossref] [PubMed]
- Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9. [Crossref] [PubMed]
- Hummel SL, Kitzman DW. Update on heart failure with preserved ejection fraction. Curr Cardiovasc Risk Rep 2013;7:495-502. [Crossref] [PubMed]
- Bossone E, D'Andrea A, D'Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr 2013;26:1-14. [Crossref] [PubMed]
- Upadhya B, Kitzman DW. Heart Failure with Preserved Ejection Fraction in Older Adults. Heart Fail Clin 2017;13:485-502. [Crossref] [PubMed]
- van Beek JH. Effects of hypoxia and hypercapnia on cardiac contractility and energetics. In: Dahan A, Teppema L, van Beek JH, editors. Physiology And Pharmacology of Cardio-Respiratory Control. Springer Netherlands; 1998:19-24.
- Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998-1005. [Crossref] [PubMed]
- Benjamin JA, Lewis KE. Sleep-disordered breathing and cardiovascular disease. Postgrad Med J 2008;84:15-22. [Crossref] [PubMed]
- Srivastava PM, Burrell LM, Calafiore P. Lateral vs medial mitral annular tissue Doppler in the echocardiographic assessment of diastolic function and filling pressures: which should we use? Eur J Echocardiogr 2005;6:97-106. [Crossref] [PubMed]
- Kamenicky P, Redheuil A, Roux C, et al. Cardiac structure and function in Cushing's syndrome: a cardiac magnetic resonance imaging study. J Clin Endocrinol Metab 2014;99:E2144-53. [Crossref] [PubMed]


