
Upper lobe preservation in the treatment of centrally located NSCLC: one more challenge in lung-sparing surgery?
Pioneer surgeons in the 1950s initially designed bronchoplastic surgery to manage patients’ poor pulmonary function, unable to tolerate pneumonectomy (1).
Nowadays, the paradigms have evolved, and gaining interest are surgical techniques that allow parenchyma preservation in the treatment of non-small cell lung cancer (NSCLC) in fit-for-surgery patients. For centrally located tumors, defined as central infiltrating NSCLC in contact with hilar structures (2), the use of technical artifice to avoid pneumonectomy is spreading.
Single sleeve lobectomy (SSL) corresponds to the resection of one lobe with standard bronchoplasty, i.e., re-implantation of the bronchus intermedius after a right upper lobectomy; or of the left inferior bronchus after a left superior lobectomy, or of the left superior bronchus after a left lower lobectomy. The superiority of sleeve lobectomy over pneumonectomy has been established in multiple publications (3,4).
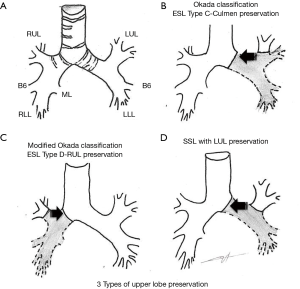
Extended-sleeve lobectomy (ESL) (5-9) corresponds to the resection of more than one lobe, with atypical bronchoplasty. Three types have been described by Okada et al. (5), and type D was added to the existing standard classification (6). Type A (5) corresponds to the re-implantation of the right lower bronchus or the right basal segment bronchus in the right main bronchus. Type B (5) refers to the re-implantation of the left basal segmental bronchus in the left main bronchus. Type C (5) refers to the anastomosis of the culminal bronchus in the left main bronchus. Type D (6) corresponds to the anastomosis of the right upper lobe bronchus in the right main bronchus after inferior bilobectomy.
SSL and ESL lobectomy can be associated with angioplasty of the pulmonary artery, such as lateral resection or resection-anastomosis or replacement, in a last-ditch effort for parenchyma preservation (10).
We have already addressed the advantages and the technical issues of single and extended sleeve lobectomy (11).
The choice of one or the other techniques partly relies on pre-operative work-up (CT-scan +/− MRI and fiber optic endoscopy). Only per-operative ascertainment can confirm the choice while waiting for a better prediction of the extension of the resection; pneumonectomy is still an option in the case of pre-operative under-estimation of the extension of the lesion, or a positive margin in per-operative frozen-section analysis.
Techniques sparing the upper lobes are seldom encountered in the literature (Table 1), as it is indicated in the very peculiar case where the tumor invades the main bronchus or its division with the upper lobe, and lower lobe or main bronchus and truncus intermedius, but neither the origins of the upper lobe, nor the proximal portion of the fissure.
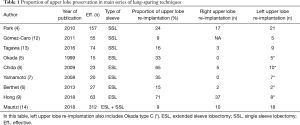
Full table
The article we comment on (14) is the first to assess specifically the operative and oncological outcome of upper lobe bronchus re-implantation. The team, expert in the domain of parenchyma preservation (15,16), combined under the term of Y-sleeve lobectomy the re-implantation of the upper lobe bronchus after an inferior left lobectomy (SSL) or an inferior right bilobectomy (ESL type D). ESL type C cases were not included in the study (Figure 1).
Over a period of more than 25 years [1989–2015], Maurizi et al. report on 28 cases of Y-sleeve lobectomy, which represented 9% of the sleeve lobectomies they performed in the same period. Eighteen were left upper lobe re-implantations (standard SSL), and 10 right upper lobe re-implantations (ESL type D).
Pre-operative work-up comprised a systematic fiber-optic endoscopy immediately before surgery, and an injected CT-scan. The authors used PET-TDM as soon as it became available in their center in 2004, but only in cases where the “presence of metastases was in doubt”. There was no systematic invasive assessment of mediastinal lymph nodes. Videomediastinoscopy was performed in the case where mediastinal or subcarinal lymph nodes appeared greater than 1.5 cm on the CT-scan. Patients treated with neo-adjuvant chemotherapy had no systematic invasive re-evaluation of lymph-node status at the end of the treatment.
Operability work-up combined clinical evaluation, a respiratory function test, blood gas analysis and perfusion lung scintigraphy in some cases. The mean predicted pre-operative forced expiratory volume in 1 s was 90.2%±14.8% (range, 61.9–115.8%).
Regarding surgical management, the authors described the main technical issues shared by upper lobe preservations (Figure 1). One key point of most sleeve resections is the necessity to preserve good vascularization of proximal and distal bronchial stumps. To do so, minimal peri bronchial dissection of the distal stump and conservation of a short mainstem bronchus stump is fairly consensual to reduce anastomotic complications. In this series, Maurizi et al. divided the right mainstem bronchus immediately above the origin of the upper lobe bronchus. On the one hand, one can expect a higher risk of bronchial fistula related to the poor vascularization of a long mainstem bronchus stump; on the other hand, direct anastomosis of the distal mainstem bronchus and the origin of upper lobe bronchus would result in a simplified management of stump caliber and shape discrepancy.
To the best of our knowledge this is the first expert surgical team in lung sparing techniques that did not report any artifice to deal with bronchial mismatch. The team performed interrupted absorbable suture, beginning at the junction between the cartilage and the membranous portion of the bronchi, and adjusting point positions.
Many teams reported cornerstone technical principles to manage bronchial anastomosis:
- Accurate millimetric distal bronchial stump dissection avoiding electrocoagulation, and the careful preservation of bronchial arteries that supply the bronchial tree is necessary.
- Caliber discrepancy was overcome by using technical adjustments to achieve a substantial narrowing of the proximal bronchial diameter.
- Traction sutures were inserted into stumps and gently pulled to reduce tension when tying anastomotic sutures; they are also used as landmarks to prevent bronchial twist.
- ‘End-to-end’ anastomosis is carried out using a hybrid technique. A continuous running 3/0 absorbable monofilament suture is applied at the deep part of anastomosis. The remaining part of anastomosis is performed using interrupted sutures, and all knots are tied outside.
Maurizi et al. (14) consider the management of the bronchial axis and the avoidance of bronchus twisting to be the greatest challenges in Y-sleeve lobectomy. We consider that both have to be anticipated at the time of the section of the bronchus, by cutting the main bronchus transversally, and using oblique bronchiotomy for the upper lobe bronchus, respecting the trifurcation of the right upper lobe bronchus and the lingual bifurcation on the left side.
The management of the tension of the anastomosis is not an issue for upper lobe re-implantation compared to other sleeve resections, and there is no use in performing any specific releasing manoeuver of the remaining lobes.
In their series, no associated pulmonary artery angioplasty was performed. In a previous report double reconstruction was most frequently carried out in type A procedures (81%) (6) and at a 50% rate in upper lobe sparing procedures. Indeed, the close proximity of the pulmonary artery and the bronchial tree at this level of the hilum promotes some extension of the centrally located NSCLC around the artery, requiring angioplasty of the pulmonary artery.
Despite these technical difficulties, the post-operative mortality rate was on the order of 3.6% (n=1), and morbidity was 25% (n=7) with only three major complications (one myocardial infarction, one bronchopleural fistula, and one anastomotic stenosis). The level of post-operative complications was comparable to that of the other series (4). The only patient who died was said to have been “unfit for pneumonectomy” with no other specific characteristics precluding this intervention. To this day, there is no available publication or recommendation that indicates sleeve lobectomy in patients unfit for pneumonectomy because of respiratory function impairment. Pneumonectomy is still an option when parenchyma sparing techniques for centrally located tumors are planned, in the case of positive margins on per-operative frozen section analysis.
All the resections were R0, but 6 patients (25%) were stage IIIA at the final pathological analysis, with all having N2 status. Nine patients received an adjuvant treatment.
They described no bronchial recurrence after a mean follow-up of 46 months (range, 2–117 months).
The global recurrence rate was 32%. The five-year overall survival rate was reported at 83.3% for stage I, 55.6% for stage II and 0% for stage IIIA NSCLC. Those results are comparable to those of other sleeve lobectomy or pneumonectomy series (3).
Five-year overall and disease-free survival rates were 0% for the 6 IIIA/N2 patients, with two of them having received induction therapy. This present finding consolidates the findings of previous, multiple studies on survival after sleeve-resection: N2 status is a powerful predictor of poor survival (17). Both this high proportion of N2 disease (25%) for a surgical series and the low proportion of diagnosis of N2 status before surgery (2/6) give credit to the recommendation of the European Society of Thoracic Surgeons (18), which advises systematic invasive exploration (endobronchial ultrasonography or video-mediastinoscopy) for centrally located lesions, whether or not there are suspicious lymph nodes on the pre-operative CT +/− PET-scan. N2 patients require particular attention and very specific treatment protocols, and surgery should not be intended in the case of N2 persisting disease after induction treatment. Systematic invasive mediastinal re-evaluation should be part of the pre-surgical work-up.
Despite its heterogeneity, its limited number of patients included over a wide study period and its retrospective nature, this series is the first one to give specific post-operative and long-term survival information about Y-sleeve lobectomy.
The report confirms the reliability of sleeve resection compared to pneumonectomy in the treatment of proximal NSCL in a global policy of parenchyma preservation, with satisfying oncological results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thomas CP. Conservative resection of the bronchial tree. J R Coll Surg (Edinb) 1956;1:169-86. [PubMed]
- Casal RF, Vial MR, Miller R, et al. What exactly is a centrally located lung tumor? Results of an online survey. Ann Am Thorac Soc 2017;14:118-23. [Crossref] [PubMed]
- Deslauriers J, Gregoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: A comparative analysis of survival and sites of recurrences. Ann Thorac Surg 2004;77:1152-6. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Extended sleeve lobectomy for lung cancer: The avoidance of pneumonectomy. J Thorac Cardiovasc Surg 1999;118:710-3. [Crossref] [PubMed]
- Berthet JP, Paradela M, Jimenez MJ, et al. Extended sleeve lobectomy: One more step toward avoiding pneumonectomy in centrally located lung cancer. Ann Thorac Surg 2013;96:1988-97. [Crossref] [PubMed]
- Yamamoto K, Miyamoto Y, Ohsumi A, et al. Sleeve lung resection for lung cancer: Analysis according to the type of procedure. J Thorac Cardiovasc Surg 2008;136:1349-56. [Crossref] [PubMed]
- Chida M, Minowa M, Miyoshi S, et al. Extended sleeve lobectomy for locally advanced lung cancer. Ann Thorac Surg 2009;87:900-5. [Crossref] [PubMed]
- Hong TH, Cho JH, Shin S, et al. Extended sleeve lobectomy for centrally located non-small-cell lung cancer: A 20-year single-centre experience. Eur J Cardiothorac Surg 2018;54:142-8. [Crossref] [PubMed]
- Berthet JP, Boada M, Paradela M, et al. Pulmonary sleeve resection in locally advanced lung cancer using cryopreserved allograft for pulmonary artery replacement. J Thorac Cardiovasc Surg 2013;146:1191-7. [Crossref] [PubMed]
- Cohen C, Berthet JP. Extended-sleeve lobectomy: A technically demanding last-ditch effort in lung-sparing surgery for central tumor. J Thorac Dis 2018;10:S2211-4. [Crossref] [PubMed]
- Gómez-Caro A, Garcia S, Reguart N, et al. Determining the appropriate sleeve lobectomy versus pneumonectomy ratio in central non-small cell lung cancer patients: An audit of an aggressive policy of pneumonectomy avoidance. Eur J Cardiothorac Surg 2011;39:352-9. [Crossref] [PubMed]
- Tagawa T, Iwata T, Nakajima T, et al. Evolution of a lung-sparing strategy with sleeve lobectomy and induction therapy for non-small cell lung cancer: A 20-year experience at a single institution. World J Surg 2016;40:906-12. [Crossref] [PubMed]
- Maurizi G, Ciccone AM, Vanni C, et al. Reimplantation of the upper lobe bronchus after lower sleeve lobectomy or bilobectomy: Long-term results. Eur J Cardiothorac Surg 2018;53:1180-5. [Crossref] [PubMed]
- D’Andrilli A, Maurizi G, Ciccione AM, et al. Long segment pulmonary artery resection to avoid pneumonectomy: Long-term results after prosthetic replacement. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [PubMed]
- Maurizi G, D’Andrilli A, Venuta F, et al. Bronchial and arterial sleeve resection for centrally-located lung cancers. J Thorac Dis 2016;8:S872-81. [Crossref] [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve-lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, Lardinois D, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]


