
Left mediastinal node dissection after arterial ligament transection via video-assisted thoracoscopic surgery for potentially advanced stage I non-small cell lung cancer
Introduction
Systematic mediastinal lymph node dissection (LND) or sampling of non-small cell lung cancer (NSCLC) is strongly recommended in published guidelines (1,2). However, mediastinal LND around the carina is anatomically challenging because mediastinal structures conceal the left paratracheal lymph nodes (stations 2L and 4L) and subcarinal lymph nodes (station 7).
Here, we describe the thoracoscopic procedures and surgical results of extended mediastinal LND involving transection of the arterial ligament for treatment of potentially node-positive left NSCLC. This technique offers a wide thoracoscopic view of the bilateral paratracheal and subcarinal spaces and facilitates detection of lymph node micrometastases in selected patients with cancer of the left lung and potential node involvement.
Methods
Study design and patients
This retrospective study was conducted at Jichi Medical University, Tochigi, Japan, and Jichi Saitama Medical Center, Saitama, Japan, from September 2008 through November 2015. Data were collected from the medical records of 75 patients who had undergone arterial ligament transection (ALT) during mediastinal systematic nodal dissection via video-assisted thoracoscopic surgery (VATS) for potentially node-positive clinical stage I NSCLC. This study was approved by the institutional review board of Jichi Medical University (No. 17-056). Informed consent was obtained in the form of opt-out on a website for the study.
Surgical indications
The patients with clinical stage I NSCLC, as categorized by the criteria included in the 7th Edition of the International Association for the Study of Lung Cancer (3) were enrolled in this study. Clinical stage was determined on the basis of preoperative chest computer tomography (CT) findings. All patients underwent positron emission tomography (PET)-CT before surgery. Potentially node-positive stage I NSCLC was diagnosed when at least one of the following factors was present:
- The standardized uptake value (SUV) of 18F-fluorodeoxyglucose in the primary tumor was greater than 3 on preoperative PET (n=63).
- The SUV of ipsilateral nodes was higher than that of contralateral nodes (n=12). Invasive biopsy via bronchoscopy was not performed because the longest diameter of lymph nodes was less than 7 mm.
- Preoperative serum carcinoembryonic antigen (CEA) concentration was greater than the normal cut-off value of 5 ng/dL (n=22).
- Hilar or mediastinal metastasis was suggested by intraoperative pathological analysis (n=28).
Surgery
Patients were placed in a right lateral decubitus position. All surgeries were successfully performed by 5-port-access thoracoscopic surgery with a rigid 45° scope. After anatomical lung resection (segmentectomy or lobectomy) and systematic LND of the hilar, mediastinal lymph node stations 3a, 5, and 6 (zone 1), the arterial ligament was exposed and dissected with an ultrasonic energy device. The main operator performed en bloc dissection of the lymph nodes around the carina (zone 2: stations 2L, 4L, and 7) while an assistant maintained a wide aorticopulmonary window (Figure 1).

Pathological diagnosis
The numbers of dissected and metastatic lymph nodes were counted by the surgeons and pathologists. Pathological diagnoses were made by at least two pathologists. TNM staging was determined by using criteria specified in the 7th Edition of the International Association for the Study of Lung Cancer.
Statistical analysis
Statistical analyses were performed with the SPSS statistical software package (version 25.0, IBM, Armonk, NY, USA). Differences between groups were evaluated with the two-sample t-test, for continuous variables, and the Chi-square test, for categorical variables. Overall survival (OS) was evaluated by Kaplan-Meier analysis, and differences between groups were analyzed with the log-rank test. A P value of 0.05 or less was considered to indicate statistical significance.
Results
Surgical results
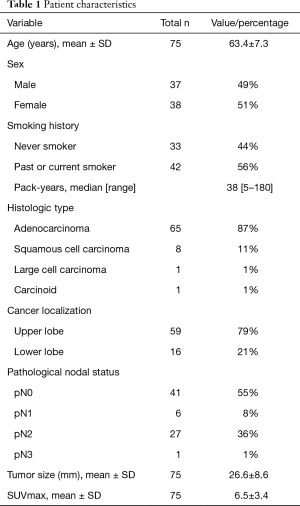
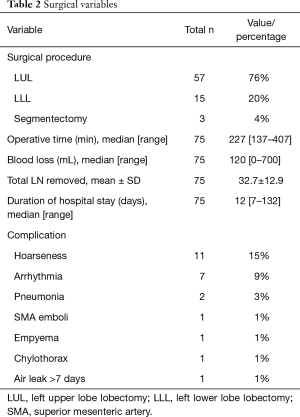
Thirty-seven men and 38 women were included in the analysis (mean age 63.4±7.3 years) (Table 1). Left upper lobectomy was the most frequent surgical procedure performed (n=57), followed by left lower-lobe lobectomy (n=15) and segmentectomy (n=3). Mean operative time was 238±58 minutes and intraoperative blood loss was 179±162 mL (Table 2). There were no intraoperative complications related to ALT. Twenty-four postoperative complications occurred in 19 patients. The most frequent was hoarseness due to recurrent nerve paralysis (n=11), followed by arrhythmia (n=7), pneumonia (n=2), emboli of the superior mesenteric artery (n=1), empyema (n=1), chylothorax (n=1), and prolonged air leak (n=1). There were no in-hospital deaths (Table 3). Eight of the 11 patients (72.7%) with hoarseness recovered during follow-up (Table 2). Routine bronchoscopy was performed for every patient on 7th postoperative day, because ischemic bronchitis was at high risk for extended LND. Thus, median duration of hospitalization was 12 days. Duration of hospitalization was 33, 75, and 132 days for patients with empyema, pneumonia, and emboli of the superior mesenteric artery postoperatively, respectively.
Full table
Full table
Full table
Pathological results
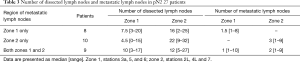
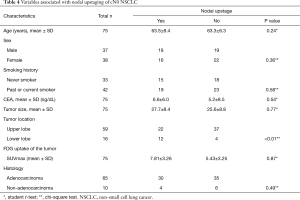
The pathological diagnoses were adenocarcinoma (n=65), squamous cell carcinoma (n=8), large-cell carcinoma (n=1), and typical carcinoid tumor (n=1). An average of 32.7±12.9 hilar and mediastinal lymph nodes were dissected. Lymph node metastasis was detected in 34 patients (6 classified as pN1; 27 as pN2). Metastasis to the right paratracheal lymph nodes was noted in 1 patient (with pN3 cancer). The average number of metastatic lymph nodes was 5.00±5.05 in node-positive patients (pN1, 1.33±0.51; pN2, 5.81±5.34; pN3, 5). Among the 27 patients with pN2 cancer, mediastinal lymph node metastasis was present at zone 2 in 10, at zone 1 in 8, and in both zones in 9 (Table 3). Nodal upstaging rate, in relation to reason for classification as potentially advanced NSCLC, were (I) primary tumor SUV >3 (52.3%); (II) lymph node laterality by PET-CT (58.3%); (III) preoperative CEA >5 ng/dL (77.2%); and (IV) suspected nodal metastasis during surgery (89.2%). Furthermore, nodal upstaging was more frequent for tumors located in the left lower lobe (P=0.03) (Table 4).
Full table
Outcomes
Median duration of follow up was 52 months, and 32 (42.7%) patients received adjuvant chemotherapy. Oral 5-fluorouracil was administered to 9 pathological stage I patients and to 1 p-stage II patient. The other 22 patients (1, 4, and 17 patients with p-stage I/II/III cancer) received platinum-doublet chemotherapy. Recurrence or metastasis during follow-up was noted in 29 patients (8 pN0 patients, 2 pN1 patients, 18 pN2 patients, and 1 pN3 patient). Only 2 patients developed locoregional lymph node recurrence around the carina.
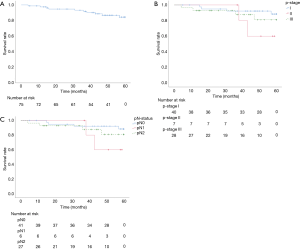
OS rates at 3 and 5 years were 91.3%/81.5% (Figure 2A). Survival at 3 and 5 years, stratified by p-stage, was 92.2%/88.4% for p-stage I, 100.0%/60.0% for p-stage II, and 87.7%/81.0% for p-stage III, respectively (Figure 2B). Survival at 3 and 5 years, stratified by regional nodal staging, was 92.2%/88.4% for pN0, 100.0%/60.0% for pN1, and 87.4%/80.7% for pN2 (Figure 2C).
Discussion
VATS is a common surgical strategy for small node-negative lung cancers. Some previous studies reported that outcomes for these cancers under VATS were not inferior to those for open thoracotomy, even when node involvement was unexpected. These include small clinical N0, pN2 lung cancers. Such cancers are associated with better outcomes than clinical and pathological N2 cancers, for which surgery is contraindicated (5). Some of the present patients might have been classified as clinical N2 cases if they had undergone invasive node biopsy via bronchoscopy or mediastinoscopy. Diagnostic accuracy of invasive biopsy for PET-positive lymph node is low even when the lymph nodes are over 1 cm (6), so necessity of preoperative invasive evaluation of PET-positive small (<1 cm) lymph node is controversial. Therefore, invasive biopsy was not performed for the present patients, even when PET-CT suggested node involvement.
The greatest obstacles in predicting surgical outcome after LND are that node involvement cannot always be definitively diagnosed without systemic LND and that stage migration substantially affects prognosis. Some previous studies found no difference in survival between lymph node sampling and systematic LND (7-9) in node-negative patients. In this study we noted that, in addition to being beneficial for precise diagnosis of nodal micrometastases in potentially node-positive NSCLC, systematic LND also yielded outcomes that were much better than those for node-positive patients in previous reports. Extended LND may improve outcomes for patients with early lung cancer and radiologically undetectable nodal micrometastases.
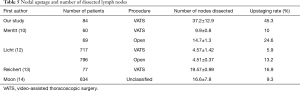
Surgical procedures, and thus stage migration, vary by center. Using VATS, Merritt et al. reported dissection of 9.9±0.8 nodes for early-stage NSCLC (10). Similarly, Denlinger et al. reported dissection of 8.9±5.2 nodes with VATS (11). As compared with techniques used in previous studies, the present ALT technique enables en bloc dissection of three times as many lymph nodes, which increased the nodal upstaging rate (Table 5) (10,12-14). Dissection of fewer than 11 lymph nodes is associated with a poor prognosis (15); thus, extended mediastinal LND might improve outcomes for left NSCLC with micrometastases. However, adapting LND via ALT is not practical for all patients with left NSCLC. Hattori et al. reported that presence of solid carcinoma, a CEA level >5 ng/dL, and a maximum SUV >5 predicted unexpected nodal involvement in clinical stage I NSCLC (16). Extended mediastinal LND under ALT might be suitable for these potentially node-positive patients.
Full table
Lymphadenectomy for the left thorax is often technically difficult because the thoracic aorta, left main pulmonary artery, and arterial ligament conceal the paratracheal and subcarinal spaces. This deep narrow space is difficult to see and limits sharing of information between surgeons under direct view via open thoracotomy. However, when using our ALT technique via VATS, this deep concealed space is readily visualized and shared by video monitor, which enables easier en bloc dissection of the carinal zone (paratracheal and subcarinal lymph nodes), as shown in Figure 1. Nodal upstaging after extended LND was more frequent in patients with cancer of the left lower lobe, because node involvement around the carina through station 7 was susceptible to metastasis (17).
The incidence of recurrent nerve paralysis in the present patients was comparatively high (14.6%). Most patients with recurrent nerve paralysis were treated in the first half of the study period, and thermal damage to the recurrent nerve by an ultrasonic energy device was the main reason for paralysis. However, 72.7% of the patients with hoarseness recovered. The energy device was not used during dissection around the recurrent nerve in the second half of the study period, as confirmed in Figure 1.
This study had some limitations, the most important of which is that it was a one-arm retrospective study at a single institution and was therefore unable to assess the true effectiveness of extended LND via ALT. A second limitation is that the stage migration effect could have affected rates of nodal upstaging and survival, because ALT was performed for selected patients with suspected advanced cancer. Despite these limitations, the excellent survival rate among node-positive patients suggests that extended LND with ALT is beneficial when radiologically negative but potentially node-positive cancer is diagnosed during surgery. Prospective clinical studies are necessary in order to clarify the survival effect of extended LND via ALT by analysis of LND, with or without ALT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Jichi Medical University (No. 17-056). Informed consent was obtained in the form of opt-out on a website for the study.
References
- Ettinger DS, Bepler G, Bueno R, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw 2006;4:548-82. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Shibano T, Tsubochi H, Tetsuka K, et al. Left mediastinal node dissection after arterial ligament transaction via video-assisted thoracoscopic surgery (the top of images shows the cranial side). Asvide 2018;5:931. Available online: http://www.asvide.com/article/view/29193
- Zhong C, Yao F, Zhao H. Clinical outcomes of thoracoscopic lobectomy for patients with clinical N0 and pathologic N2 non-small cell lung cancer. Ann Thorac Surg 2013;95:987-92. [Crossref] [PubMed]
- Lilo MT, Allison DB, Younes BK, et al. The critical role of EBUS-TBNA cytology in the staging of mediastinal lymph nodes in lung cancer patients: A correlation study with positron emission tomography findings. Cancer Cytopathol 2017;125:717-725. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Wu N, Yan S, Lv C, et al. Does an extended mediastinal lymphadenectomy improve outcome after R0 resection in lung cancer? Chin J Cancer Res 2014;26:183-91. [PubMed]
- Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290-4; discussion 294-5. [Crossref] [PubMed]
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [Crossref] [PubMed]
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5; discussion 1736.
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Reichert M, Steiner D, Kerber S, et al. A standardized technique of systematic mediastinal lymph node dissection by video-assisted thoracoscopic surgery (VATS) leads to a high rate of nodal upstaging in early-stage non-small cell lung cancer. Surg Endosc 2016;30:1119-25. [Crossref] [PubMed]
- Moon Y, Lee KY, Kim KS, et al. Clinicopathologic correlates of postoperative N1 or N2 nodal upstaging in non-small cell lung cancer. J Thorac Dis 2016;8:79-85. [PubMed]
- Tantraworasin A, Saeteng S, Siwachat S, et al. Impact of lymph node management on resectable non-small cell lung cancer patients. J Thorac Dis 2017;9:666-74. [Crossref] [PubMed]
- Hattori A, Suzuki K, Matsunaga T, et al. Is limited resection appropriate for radiologically "solid" tumors in small lung cancers? Ann Thorac Surg 2012;94:212-5. [Crossref] [PubMed]
- Shimada Y, Saji H, Kakihana M, et al. Retrospective analysis of nodal spread patterns according to tumor location in pathological N2 non-small cell lung cancer. World J Surg 2012;36:2865-71. [Crossref] [PubMed]


