
The gender-specific expression of neuropeptide Y and neuropeptide Y receptors in human atrial tissue during cardiopulmonary bypass surgery
Introduction
Myocardial ischemia-reperfusion (I/R) injury is a well-recognized pathophysiological process, which occurs in several clinical conditions such as infarction, shock, organ transplantation and cardiopulmonary bypass (CPB) surgery (1). Several pathogenetic mechanisms involved have been explored. Among them, the imbalance between sympathetic and parasympathetic system inputs to the heart, is one of the major reasons which induce myocardial ischemia, arrhythmia and death (2).
Neuropeptide Y (NPY) is a 36-residue peptide amide, co-stored and co-released with noradrenaline in sympathetic nerve fibers of postganglionic neurons innervating the cardiovascular system. NPY is associated with hypertension (3), myocardial infarction (4), cardiomyopathy (5), suggesting that NPY functions in cardiovascular homeostasis. To date, six NPY receptors (NPYRs), namely NPYRs Y1–Y6 have been reported (6,7). NPYRs regulate the biological processes through diverse ways. NPY Y1 promoted cellular shortening and calcium transient in rat ventricular myocytes (8). Antagonists of NPY Y2 could prevent the inhibition of vagal bradycardia after sympathetic stimulation (9). The influence of vagal neurotransmission and ventricular myocyte excitability was accomplished even in the presence of β-receptor blockade, which indicated that pharmacologically targeting NPYRs might be a useful therapeutic strategy synergistically with β-receptor blockers (10). Moreover, the expression of NPY was increased in human plasma after CPB (11), implying NPY signaling controlled the pathological progression of CPB. However, the expression changes of NPY and NPYRs in human heart itself during CPB surgery remain elusive. Whether the expression of NPY and NPYRs have any correlations with clinical data needs to be investigated.
Methods
Collection of specimens
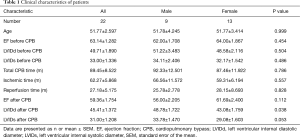
Patients were hospitalized for cardiac surgery in Anzhen Hospital, Beijing. According to preoperative Doppler echocardiography, rheumatic mitral stenosis or insufficiency were found in these patients, partly with tricuspid insufficiency. Patients with cerebrovascular accidents, diabetes mellitus, myocardial infarction and other diseases were excluded. All researches involving human participants were approved by Medical Ethics Committee. Written informed consent was obtained from the patients and data were analyzed anonymously. Patient characteristics were summarized in Table 1. All patients underwent superior vena cava catheterization in the right atrial appendage. A few specimens were taken from the right atrium during the intubation of the superior vena cava (before CPB) and the extubation of the superior vena cava (after CPB), which were routinely excised and discarded tissue. The specimens were stored in RNA stabilization reagent (Qiagen) for later analysis.
Full table
Total RNA extraction of patients’ atrium
Trizol was used to extract the total RNAs from patients’ atrium. Trizol (sigma) was added in patients’ atrial tissue for lysis, then chloroform and isopropanol were used for purification, finally the supernatant of centrifugation was dissolved in water with RNase free, and stored at −80 °C.
Reverse transcription of mRNA
The total mRNA from patients’ atrium was reversely transcribed according to SuperScript™ III RT kit (Invitrogen). Appropriate amount of freshly extracted RNAs with Primer, dNTP mix, SuperScript III RT were used. The resulting cDNAs were preserved at −20 °C.
Real-time quantitative polymerase chain reaction (RT-qPCR)
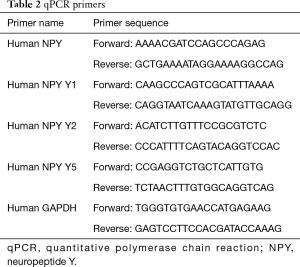
RT-qPCR was carried out with SYBR Green (ABI). The total reaction system was 20 µL, 50 °C 2 min, 95 °C 10 min, 40 cycles for 95 °C 15 sec, 60 °C 1 min. GAPDH was set as the internal parameter and the relative mRNA levels were calculated with the 2−ΔΔCt method. The primers were listed in Table 2.
Full table
Statistical analysis
Results were presented as means ± standard error of the mean (SEM) or median (interquartile range). Data were evaluated by one-way ANOVA or nonparametric tests. A value of P<0.05 was considered statistically significant.
Results
General information
Twenty-two patients hospitalized for cardiac surgery due to rheumatic heart disease were randomly enrolled. These patients received cardiac valve replacement surgery by CPB. The mean age of all patients was 51.77±2.597, total CBP time was 89.45±8.522 min, ischemic time was 62.27±5.868 min, reperfusion time was 27.18±5.175 min. There was no statistically difference between male and female patients in such general information. The cardiac functions such as ejection fraction (EF), left ventricular internal diastolic diameter (LVIDd), left ventricular internal systolic diameter (LVIDs) were detected by echocardiography before and after CPB. After surgery, the patients’ cardiac functions were partly recovered, such as LVIDd returned to normal range (Table 1).
The expression of NPY and NPYRs in human atrium before and after CPB surgery
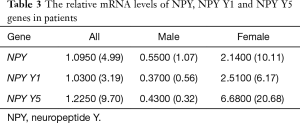
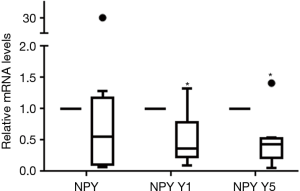
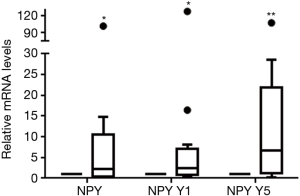
Using RT-qPCR, the transcriptional levels of NPY and NPY receptor genes were analyzed. We found NPY and NPY Y1, Y2, Y5 genes could express in human atrium, of them, the transcriptional level of NPY Y2 was the least. The mRNA levels of NPY, NPY Y1 and NPY Y5 were compared before and after CPB in each patient (Table 3). For all 22 patients, the relative mRNA levels of NPY, NPY Y1, NPY Y5 were 1.0950 (4.99) (P=0.236), 1.0300 (3.19) (P=0.069) and 1.2250 (9.70) (P=0.149) respectively. There was no statistically difference before and after CPB in all patients. But the relative mRNA levels of NPY Y1 [0.3700 (0.56), P=0.021] and NPY Y5 [0.4300 (0.32), P=0.011] were statistically down-regulated in male patients after CPB (Figure 1). On the contrary, the relative transcriptional levels of NPY [2.1400 (10.11), P=0.039], NPY Y1 [2.5100 (6.17), P=0.019] and NPY Y5 [6.6800 (20.68), P=0.004] were statistically up-regulated in female patients after CPB (Figure 2). These results implied that NPY and NPYRs might play a role in CPB surgery, although the mechanism could be different between male and female patients.
Full table
Correlation analysis
The correlation between patients’ clinical cardiac functions (LVIDd, LVIDs, EF before and after CPB), CPB surgery time (ischemia time, reperfusion time), and the transcriptional levels of NPY, NPY Y1, NPY Y5 were all analyzed. There was only a significant negative correlation between cardiac EF after CPB and the relative mRNA levels of NPY (Spearman’s correlation coefficient =−0.706, P=0.034) in male patients. There was no correlation in other factors.
Discussion
CPB needs to block the cardiac blood flow first, myocardial oxygenation is restored after myocardial ischemia for a short period, and ischemia reperfusion injury happens simultaneously (1). In this process, the cardiac function is regulated by the autonomic nervous system. It is generally accepted that the activated sympathetic system and the suppressed parasympathetic system contribute to the increased blood pressure, myocardial oxygen consumption and cerebrovascular accidents (2). The sympathetic system mainly releases norepinephrine (NE) and activated β adrenergic receptors to increase heart rate and myocardial contraction. Antagonistically, the parasympathetic system release acetylcholine (ACh) and activated muscarinic ACh receptors on the opposite way.
It has been proved that NPY regulates NE release in the terminal of sympathetic fibers. The NPY signaling plays comprehensive roles in all typed cardiac cells. In human right ventricular endocardial endothelial cells, NPY Y1 modulates the cytosolic and nuclear calcium homeostasis, and their secretion of NPY (12). In cardiomyocytes, NPY had been shown to stimulate hypertrophy of rat and chick cardiomyocytes through activation of NPY Y1 receptor (13,14). Pellieux et al. found that NPY via NPY Y5 receptor participated in the development of mouse cardiac hypertrophy through MAPK cascade (15). These data suggest NPY Y1 and Y5 have positive chronotropic effects on ventricular cardiomyocytes. With respect to NPY Y2 receptor, it reduces ACh release and vagal bradycardia after sympathetic stimulation (16). These data mentioned above suggest that pharmacologically targeting NPYRs might conduce to cardiovascular homeostasis.
To our knowledge, this is the first reports that the transcriptional levels of NPY and NPYRs genes were investigated in atrial samples from patients with rheumatic diseases before and after CPB surgery. Using qPCR, we found that NPY, NPY Y1, NPY Y2 and NPY Y5 were all expressed in human right atrium, which was consistent with Ejaz’s study (17). But the NPY Y2 transcriptional level was so hard to detect in our patients. Since NPY Y2 receptor was thought to be a presynaptic receptor (18), its transcriptional level was little.
In all 22 patients, there was no statistically difference between the transcriptional levels of NPY, NPY Y1 and NPY Y5 before and after CPB. In spite of the sex factor, our data revealed that NPY mRNA level was up-regulated after CPB in female patients. It had been reported that patient plasma NPY concentration was elevated after CPB since cardiac sympathetic nerve activity was increased after surgery (19). In this study the up-regulation of NPY mRNA in female patients in atrium might be attributed to the elevated NPY levels in plasma. In addition, we found that, only four people had atrial fibrillation after CPB in our 22 patients, and interestingly they were all females. Guler group demonstrated that NPY played a role in the occurrence of atrial fibrillation in the patients after coronary bypass surgery (11). Hence, the atrial fibrillation in female patients might be associated with up-regulation of NPY expression. Since the number of atrial fibrillation patients was small, this manifestation needed to be investigated further. Though there was no statistically difference between the transcriptional levels of NPY in male patients before and after CPB, we found that the relative mRNA level of NPY was negative correlated with cardiac EF after CPB in these patients. Luo et al. reported that there was a dose-dependent stimulation of NPY with decreased ATP content and activity in the rat cardiomyocytes (20). Thus, our findings reinforced that the decreased level of NPY mRNA in male atriums might help the patients to recover cardiac functions after surgery.
In this study, the transcriptional levels of NPY Y1 and NPY Y5 were both up-regulated in female patients, but down-regulated in male patients after CPB, which suggested that NPY signaling was activated in female patients. Jackson et al. found that NPY Y1 expression level was higher in the male rat skeletal muscle compared with that in females (21). In our atrium muscle tissue, the expression levels of NPY Y1 and NPY Y5 were also stronger in male patients than that of female patients. Musso et al. found that estradiol could stimulate NPY Y1 transcription through estrogen receptor alpha (ER-α) (22). However, we could not find significant difference in the mRNA levels of ER-α in female patients before and after CPB (data not shown). Since ER-α moved from cytoplasm to nucleus to regulate target genes (23), the up-regulation of NPY Y1 expression in female patients might be due to the cytoplasm-nucleus translocation. NPY and its receptors had been found gender-specificity in other disease, such as type 2 diabetes (24) and severe periodontitis (25). Since targeting NPYRs pharmacologically may be a useful therapeutic strategy synergistically with β-blockers for cardiovascular diseases, the gender-specificity should be taken into account.
Conclusions
Our results suggested that there was gender-specify in the expression of NPY and NPY receptor genes during CPB surgery and NPY signaling was activated in female patients. Gender differences should be considered when using NPYRs as pharmacologically targets for the treatment of cardiovascular diseases.
Acknowledgements
We thank Professor Zhiqing Xu, Professor Liang Li from the Capital Medical University; Professor Xuejun Jiang from Chinese Academy of Sciences for their supports.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics review board of the Capital Medical University affiliated Beijing Anzhen Hospital (No. 2009001X) and written informed consent was obtained from all patients.
References
- Niemann B, Schwarzer M, Rohrbach S. Heart and Mitochondria: Pathophysiology and Implications for Cardiac Surgeons. Thorac Cardiovasc Surg 2018;66:11-9. [Crossref] [PubMed]
- Finlay M, Harmer SC, Tinker A. The control of cardiac ventricular excitability by autonomic pathways. Pharmacol Ther 2017;174:97-111. [Crossref] [PubMed]
- Baltazi M, Katsiki N, Savopoulos C, et al. Plasma neuropeptide Y (NPY) and alpha-melanocyte stimulating hormone (a-MSH) levels in patients with or without hypertension and/or obesity: a pilot study. Am J Cardiovasc Dis 2011;1:48-59. [PubMed]
- Cuculi F, Herring N, De Caterina AR, et al. Relationship of plasma neuropeptide Y with angiographic, electrocardiographic and coronary physiology indices of reperfusion during ST elevation myocardial infarction. Heart 2013;99:1198-203. [Crossref] [PubMed]
- Dvorakova MC, Kruzliak P, Rabkin SW. Role of neuropeptides in cardiomyopathies. Peptides 2014;61:1-6. [Crossref] [PubMed]
- Wahlestedt C, Grundemar L, Hakanson R, et al. Neuropeptide Y receptor subtypes, Y1 and Y2. Ann N Y Acad Sci 1990;611:7-26. [Crossref] [PubMed]
- Lecklin A, Lundell I, Paananen L, et al. Receptor subtypes Y1 and Y5 mediate neuropeptide Y induced feeding in the guinea-pig. Br J Pharmacol 2002;135:2029-37. [Crossref] [PubMed]
- Heredia MP, Delgado C, Pereira L, et al. Neuropeptide Y rapidly enhances [Ca2+]i transients and Ca2+ sparks in adult rat ventricular myocytes through Y1 receptor and PLC activation. J Mol Cell Cardiol 2005;38:205-12. [Crossref] [PubMed]
- Smith-White MA, Hardy TA, Brock JA, et al. Effects of a selective neuropeptide Y Y2 receptor antagonist, BIIE0246, on Y2 receptors at peripheral neuroeffector junctions. Br J Pharmacol 2001;132:861-8. [Crossref] [PubMed]
- Herring N. Autonomic control of the heart: going beyond the classical neurotransmitters. Exp Physiol 2015;100:354-8. [Crossref] [PubMed]
- Guler N, Ozkara C, Dulger H, et al. Do cardiac neuropeptides play a role in the occurrence of atrial fibrillation after coronary bypass surgery? Ann Thorac Surg 2007;83:532-7. [Crossref] [PubMed]
- Jacques D, D’Orléans-Juste P, Magder S, et al. Neuropeptide Y and its receptors in ventricular endocardial endothelial cells. Can J Physiol Pharmacol 2017;95:1224-9. [Crossref] [PubMed]
- Millar BC, Schluter KD, Zhou XJ, et al. Neuropeptide Y stimulates hypertrophy of adult ventricular cardiomyocytes. Am J Physiol 1994;266:C1271-7. [Crossref] [PubMed]
- Jacques D, Abdel-Samad D., Neuropeptide Y. NPY) and NPY receptors in the cardiovascular system: implication in the regulation of intracellular calcium. Can J Physiol Pharmacol 2007;85:43-53. [Crossref] [PubMed]
- Pellieux C, Sauthier T, Domenighetti A, et al. Neuropeptide Y (NPY) potentiates phenylephrine-induced mitogen-activated protein kinase activation in primary cardiomyocytes via NPY Y5 receptors. Proc Natl Acad Sci U S A 2000;97:1595-600. [Crossref] [PubMed]
- Herring N, Lokale MN, Danson EJ, et al. Neuropeptide Y reduces acetylcholine release and vagal bradycardia via a Y2 receptor-mediated, protein kinase C-dependent pathway. J Mol Cell Cardiol 2008;44:477-85. [Crossref] [PubMed]
- Ejaz A, LoGerfo FW, Khabbaz K, et al. Expression of Neuropeptide Y, Substance P, and their receptors in the right atrium of diabetic patients. Clin Transl Sci 2011;4:346-50. [Crossref] [PubMed]
- Smith-White MA, Herzog H, Potter EK. Role of neuropeptide Y Y(2) receptors in modulation of cardiac parasympathetic neurotransmission. Regul Pept 2002;103:105-11. [Crossref] [PubMed]
- Cooper TJ, Clutton-Brock TH, Jones SN, et al. Factors relating to the development of hypertension after cardiopulmonary bypass. Br Heart J 1985;54:91-5. [Crossref] [PubMed]
- Luo G, Xu X, Guo W, et al. Neuropeptide Y damages the integrity of mitochondrial structure and disrupts energy metabolism in cultured neonatal rat cardiomyocytes. Peptides 2015;71:162-9. [Crossref] [PubMed]
- Jackson DN, Milne KJ, Noble EG, et al. Gender-modulated endogenous baseline neuropeptide Y Y1-receptor activation in the hindlimb of Sprague-Dawley rats. J Physiol 2005;562:285-94. [Crossref] [PubMed]
- Musso R, Maggi A, Eva C. 17beta-estradiol stimulates mouse neuropeptide Y-Y(1) receptor gene transcription by binding to estrogen receptor alpha in neuroblastoma cells. Neuroendocrinology 2000;72:360-7. [Crossref] [PubMed]
- Sebastian T, Sreeja S, Thampan RV. Import and export of nuclear proteins: focus on the nucleocytoplasmic movements of two different species of mammalian estrogen receptor. Mol Cell Biochem 2004;260:91-102. [Crossref] [PubMed]
- Campbell CD, Lyon HN, Nemesh J, et al. Association studies of BMI and type 2 diabetes in the neuropeptide Y pathway: a possible role for NPY2R as a candidate gene for type 2 diabetes in men. Diabetes 2007;56:1460-7. [Crossref] [PubMed]
- Freitag-Wolf S, Dommisch H, Graetz C, et al. Genome-wide exploration identifies sex-specific genetic effects of alleles upstream NPY to increase the risk of severe periodontitis in men. J Clin Periodontol 2014;41:1115-21. [Crossref] [PubMed]


