
Saphenous vein graft failure: seeing the bigger picture
Approximately 50% of saphenous vein grafts (SVGs) fail by 5 to 10 years post-coronary artery bypass grafting (CABG) and between 20–40% fail within the first year (1,2). While SVG failure can sometimes be silent, when symptomatic events occur, SVG percutaneous coronary intervention (PCI) is often performed. SVG PCI represents approximately 6% of the total PCI volume in the US (3). Given the aggressiveness of SVG atherosclerosis and the high risk for recurrent SVG failure, what are the optimal prevention and treatment options in such patients?
Prevention: use of arterial grafts during CABG
One way to avoid SVG failure is to not use SVGs in the first place during CABG. However, the frequency of bilateral internal mammary artery (BIMA) grafting and of radial artery (RA) grafting during CABG remains low in the US (approximately 5% each) (2). This is at least in part due to conflicting evidence with some, but not all studies showing benefit with BIMA and RA grafts as compared with SVGs (4,5).
Both the 2011 American College of Cardiology/American Heart Association (ACC/AHA) and 2018 European Society of Cardiology/European Association of Cardio-Thoracic Surgery (ESC/EACTS) Guidelines advocate (class I) that every patient undergoing CABG should receive a left IMA graft to the left anterior descending (LAD) (4,6). The ESC/EACTS guidelines also recommend that an additional arterial graft should be considered in appropriate patients (class IIa, level of evidence B) (4). They also recommend RA over SVG grafts in patients with severe coronary stenoses (class I, level of evidence B) (4). The ACC/AHA guidelines recommend use of RA grafts in severe (>70%) left coronary artery stenoses and critical (≥90%) RCA stenoses (class IIb, level of evidence B) (6). In addition, both the ESC/EACTS and ACC/AHA guidelines recommend considering BIMA grafting (class IIa, level of evidence B) in patients who do not appear at excess risk of poor sternal healing or infection and the ACC/AHA guidelines recommend that complete arterial revascularization be considered (class IIb, level of evidence C) in individuals <60 years of age with few comorbidities (4,6).
The superiority of using the RA over SVGs is supported in a recent meta-analysis of 1,036 patients in whom RA grafts were associated with a lower rate of adverse cardiac events (HR 0.67; 95% CI: 0.49–0.90; P=0.01) and occlusion (HR, 0.44; 95% CI: 0.28–0.70; P<0.001) at 5 year follow-up as compared with SVGs (7). In a retrospective analysis of 13,324 patients, both use of RA (HR 0.82, P<0.001) and right IMA (HR 0.86, P=0.03) were associated with lower mortality as compared with SVGs in <70-year-old patients (2).
In contrast, the recently reported 10-year results from the Arterial Revascularization Trial (ART) did not demonstrate benefit with BIMA vs. single IMA grafting (https://www.escardio.org/The-ESC/Press-Office/Press-releases/ten-year-outcomes-of-the-arterial-revascularisation-trial-revealed-today) (5). In ART 1,548 patients were randomized to BIMA and 1,554 to single IMA grafts. Additional arterial or vein grafts were used at the discretion of the surgeon. At 10-year follow-up, all-cause mortality in the BIMA and single IMA groups was 20.4% and 21.2%, respectively (HR 0.96, 95% CI: 0.82–1.12, P=0.58). The 10-year incidence of the composite endpoint of death from any cause, MI, or stroke was 24.9% and 27.4% in the BIMA and single IMA groups, respectively (HR 0.90, 95% CI: 0.78–1.03, P=0.12). Outcomes were, however, more favorable in patients who had BIMA performed by more experienced surgeons. In an exploratory analysis of ART, patients who received any 2 arterial grafts (IMA or radial) appeared to have lower mortality (HR 0.79, 95% CI: 0.64–0.97) and lower incidence of the composite of death, MI or stroke (HR 0.80, 95% CI: 0.69–0.93) vs. those who had received a single arterial graft. The latter was consistent with another post-hoc analysis of the 5-year ART results, in which use of a RA graft in addition to single IMA or BIMA grafts was associated with lower risk for midterm major adverse cardiac events (8). Interpretation of the ART trial results is challenging, however, because: (I) many patients randomized to BIMA, actually received a single IMA graft and (II) many patients randomized to single IMA graft actually received an additional RA. Use of guideline-directed medications was also high, which may have limited the ability to detect differences and which may not reflect real-world compliance and average physician prescribing patterns in post-CABG patients.
The method of arterial graft harvesting and post-procedural management such as ongoing use of calcium channel blockers (CCB), could further improve arterial graft outcomes: in a study of 732 patients, ongoing CCB use was associated with better RA graft patency (HR, 0.20; 95% CI: 0.08–0.49; P<0.001) (9). Conversely, use of the “no-touch” SVG harvesting technique (harvesting with a small amount of surrounding tissue that decreases SVG injury and the need for manual distention of the SVG) has been associated with better patency (10).
Ultimately, it would seem that arterial grafting should, and could be considered in more patients, depending on the individual’s age/life expectancy, coronary anatomy, and risk for sternal wound infections.
The post-CABG patient with a patent SVG-how do we maintain patency?
When a SVG is plugged into the arterial system, its physiologic milieu changes and it undergoes adaptive remodeling, starting with fibrointimal hyperplasia (11). This hyperplasia forms the nidus for atherosclerosis formation, which is usually established by 5–10 years and is often associated with thrombus and friability (11). Although aspirin and statins have been shown to improve SVG patency, additional treatments may be beneficial (12). There has been support from two studies (Ticagrelor, PLATO; Prasugrel, TRITON-TIMI 38), for improved clinical outcomes in post-ACS patients undergoing CABG when they are treated with novel anti-platelet agents over clopidogrel (12). In a recent study of 231 prior CABG patients, higher circulating PCSK9 levels were associated with higher risk for late SVG degeneration after adjustment for established cardiovascular risk factors (13). The ongoing Alirocumab for Stopping Atherosclerosis Progression in Saphenous Vein Grafts (ASAP-SVG) Pilot Trial (https://clinicaltrials.gov/ct2/show/NCT03542110) is evaluating the effect of alirocumab vs. placebo on intermediate SVG lesion atherosclerotic disease burden as assessed by IVUS after 78 weeks of treatment.
Best practices for SVG PCI
In SVG PCI, prevention and treatment of distal embolization and no reflow are of paramount importance and could be achieved by using intragraft vasodilators and embolic protection devices (EPDs). EPD use carries a class I recommendation in the ACC/AHA PCI guidelines, but was recently downgraded to class IIa in the European Society of Cardiology guidelines, based on observational data that are, however, subject to significant bias.
Stent choice in SVG PCI was recently simplified by the Drug Eluting Stents in Saphenous Vein Graft Angioplasty (DIVA) and the long-term outcomes of the Is Drug-Eluting-Stenting Associated with Improved Results in Coronary Artery Bypass Grafts? Trial (ISAR-CABG) trial, both of which showed no benefit (but also no penalty) with use of DES in SVGs (14,15). In countries where DES are significantly more expensive than BMS, BMS should be used. In countries with similar DES and BMS prices, either is acceptable.
Prolonged dual anti-platelet therapy may also improve outcomes after SVG PCI (16). The DAPT score assigns 2 points for PCI in an SVG, which in a patient with an otherwise acceptable bleeding risk, would favor more prolonged dual anti-platelet duration (17).
The post-CABG patient with a failing SVG—alternatives to SVG PCI
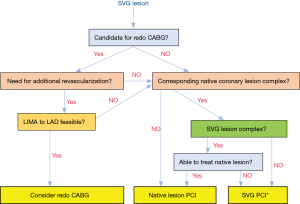
PCI of a degenerated SVG is associated with poor long-term outcomes, regardless of the strategy and technique used. PCI of the native vessel that supplies the same territory as the failing SVG is likely superior (18). However, native coronary artery PCI can be challenging in prior CABG patients due to anatomical limitations, clinical acuity, or operator skillset and equipment required. Figure 1 presents a decision tree with treatment options for failing SVGs depending on the complexity of the SVG and the corresponding native coronary artery lesion(s). In addition to acute native vessel PCI, the concept of acute SVG PCI with staged native vessel PCI (when complex) has been proposed, and may represent another avenue by which to provide more durable results, particularly in patients who have had multiple within-graft interventions (3). As operators gain more experience in complex PCI, when the risk/benefit ratio is favorable, native vessel PCI, may become an increasingly appealing option in patients presenting with SVG failure.
Acknowledgements
None.
Footnote
Conflicts of Interest: ES Brilakis: consulting/speaker honoraria from Abbott Vascular, American Heart Association (associate editor Circulation), Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), CSI, Elsevier, GE Healthcare, InfraRedx, and Medtronic; research support from Regeneron and Siemens. Shareholder: MHI Ventures. Board of Trustees: Society of Cardiovascular Angiography and Interventions. AB Hall has no conflicts of interest to declare.
References
- Taggart DP. Current status of arterial grafts for coronary artery bypass grafting. Ann Cardiothorac Surg 2013;2:427-30. [PubMed]
- Tranbaugh RF, Schwann TA, Swistel DG, et al. Coronary Artery Bypass Graft Surgery Using the Radial Artery, Right Internal Thoracic Artery, or Saphenous Vein as the Second Conduit. Ann Thorac Surg 2017;104:553-9. [Crossref] [PubMed]
- Xenogiannis I, Tajti P, Burke MN, et al. Staged revascularization in patients with acute coronary syndromes due to saphenous vein graft failure and chronic total occlusion of the native vessel: A novel concept. Catheter Cardiovasc Interv 2019;93:440-4. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:e652-735. [PubMed]
- Gaudino M, Benedetto U, Fremes S, et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N Engl J Med 2018;378:2069-77. [Crossref] [PubMed]
- Taggart DP, Altman DG, Flather M, et al. Associations Between Adding a Radial Artery Graft to Single and Bilateral Internal Thoracic Artery Grafts and Outcomes: Insights From the Arterial Revascularization Trial. Circulation 2017;136:454-63. [Crossref] [PubMed]
- Gaudino M, Benedetto U, Fremes S, et al. Effect of chronic calcium-channel blocker therapy after coronary artery bypass with the radial artery: insights from the RADIAL database. Available online: https://www.thecardiologyadvisor.com/home/conference-highlights/aha-2018-meeting-highlights/chronic-ccb-therapy-linked-to-improved-outcomes-after-cabg-utilizing-the-radial-artery/
- Souza DS, Arbeus M, Botelho Pinheiro B, et al. The no-touch technique of harvesting the saphenous vein for coronary artery bypass grafting surgery. Multimed Man Cardiothorac Surg 2009;2009:mmcts.2008.003624.
- Beijk MA, Harskamp RE. Treatment of Coronary Artery Bypass Graft Failure. Available online: https://www.intechopen.com/books/artery-bypass/treatment-of-coronary-artery-bypass-graft-failure
- McKavanagh P, Yanagawa B, Zawadowski G, et al. Management and Prevention of Saphenous Vein Graft Failure: A Review. Cardiol Ther 2017;6:203-23. [Crossref] [PubMed]
- Gao J, Wang HB, Xiao JY, et al. Association between proprotein convertase subtilisin/kexin type 9 and late saphenous vein graft disease after coronary artery bypass grafting: a cross-sectional study. BMJ Open 2018;8:e021951. [Crossref] [PubMed]
- Brilakis ES, Edson R, Bhatt DL, et al. Drug-eluting stents versus bare-metal stents in saphenous vein grafts: a double-blind, randomised trial. Lancet 2018;391:1997-2007. [Crossref] [PubMed]
- Colleran R, Kufner S, Mehilli J, et al. Efficacy Over Time With Drug-Eluting Stents in Saphenous Vein Graft Lesions. J Am Coll Cardiol 2018;71:1973-82. [Crossref] [PubMed]
- Redfors B, Généreux P, Witzenbichler B, et al. Percutaneous Coronary Intervention of Saphenous Vein Graft. Circ Cardiovasc Interv 2017.10. [PubMed]
- Kereiakes DJ, Yeh RW, Massaro JM, et al. DAPT Score Utility for Risk Prediction in Patients With or Without Previous Myocardial Infarction. J Am Coll Cardiol 2016;67:2492-502. [Crossref] [PubMed]
- Brilakis ES, O'Donnell CI, Penny W, et al. Percutaneous Coronary Intervention in Native Coronary Arteries Versus Bypass Grafts in Patients With Prior Coronary Artery Bypass Graft Surgery: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. JACC Cardiovasc Interv 2016;9:884-93. [Crossref] [PubMed]


