Gastrointestinal bleeding in lung leiomyosarcoma history: report of a case
Introduction
Primary pulmonary sarcoma is a very rare entity, accounting for less than 0.5% of all malignant lung cancers, originating from the smooth muscle of the pulmonary parenchyma, bronchi, or pulmonary arteries, in order of decreasing frequency (1). Because of their rarity, the biologic behaviour of these tumours is not well known. Metastases to the small bowel from primary tumours in extra abdominal sites are uncommon and the most common primary tumours that metastasise to the bowel are malignant melanoma, carcinoma of the breast and lung (2). Clinical features of gastrointestinal (GI) metastases are abdominal pain, bleeding, obstruction, and perforation: GI haemorrhage is the most common symptom of GI metastasis (3). Abdominal metastasis is considered an uncommon event in the natural history of sarcoma and limited information are available regarding the natural history of primary lung sarcoma, the influence of abdominal metastasis and the significance of surgical resection. To our knowledge, small bowel metastasis from primary lung leiomyosarcoma (PLL) has never previously been reported.
We present an unusual case of single small bowel metastasis from PLL presenting with abdominal pain and GI bleeding successfully treated by salvage surgery.
Material and method
A 67-year-old woman presented to our Hospital with intermittent upper-abdominal ache for common bile duct calculosis; a routine radiographic image of the chest exposed an abnormal lung shadow in the upper lobe of his right lung. Computed tomography (CT) scan of the chest noted a mass in the right upper lung base with no evidence of mediastinal lymphadenopathy (Figure 1) and positron-emission tomography (PET) ruled out extrathoracic locations of the disease. Evaluation of transbronchial needle aspiration (TBNA) specimens showed undifferentiated non small cell lung carcinoma.
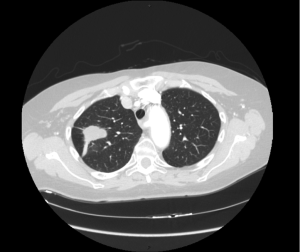
The patient presented to the thoracic surgeon and underwent right thoracotomy with upper pulmonary lobectomy and mediastinal lymphadenectomy; grossly the lesion was round and white, measuring 31 mm × 22 mm, infiltrating the visceral pleura. Surgical pathology report revealed that the lesion was composed of bundles of spindle-shaped cells, with areas of necrosis, sclerosis and hyalinosis. Immunohistochemical study revealed that tumor was positive for smooth muscle actin, CD68 but negative for CD34, CK(PAN), desmin, TTF, S100, ACS, AML, CD117, MIB-1 >20%. The lesion was diagnosed as leiomyosarcoma. Mediastinal lymphadenectomy was negative for metastatic disease.
Because the patient had no history of a resected leiomyosarcoma and no tumor was detected by clinical and diagnostic imaging studies, a diagnosis of PLL was made.
The patient did no underwent radiotherapy or chemotherapy and was on regular follow-up: whole-body PET/CT or CT scan of chest and abdomen were performed every 3 months.
Two years after surgery the patient presented abdominal pain. Upper GI endoscopy showed hiatal hernia. Fecal occult blood test was positive and colonoscopy did no detect lesions or bleeding. Following the onset of melena and worsening anemia she was admitted to hospital. Video capsule endoscopy (VCE) showed a stenosing and ulcerative ileal lesion. Double-balloon enteroscopy with biopsies of the lesion resulted positive for spindle-cell carcinoma, suggestive of leiomyosarcoma metastasis.
We performed an oncological correct laparoscopic segmental ileal resection. Pathology evaluation of the specimen verified a white, stenosing lesion, measuring 60 mm × 24 mm retracting and infiltrating serous membrane and involving the adjacent mesentery. Surgical pathology revealed high degree leiomyosarcoma, with markedly increased areas of mitotic activity, with full-thickness bowel infiltration with mucosal ulceration; lymph nodes and surgical margins of resection were negative for neoplastic disease. Immunohistochemical study revealed that tumor was positive for smooth muscle actin, desmin but negative for CD34, CK(PAN), DOG1, S100, AML, CD117, MIB-1 >20%. The lesion was diagnosed as lung leiomyosarcoma metastasis.
Results
The postoperative course was uneventful. The patient received adjuvant chemotherapy and is still alive without recurrence for >3 years.
Discussion
Leiomyosarcoma represent a heterogeneous group of tumors and usually arises in the uterus, GI tract, retroperitoneum or soft tissue accounting for 1% of all malignancies. Pulmonary leiomyosarcoma is a rare disease representing 9% of all sarcomas (4), originating from the neoplastic transformation of the peribronchial smooth muscle fibers, most frequently the larger bronchi of the left lower lobe (5). Risk factors include radiation therapy, chemotherapy and environmental and occupational exposures. Most primary sarcomas of the lung occur in middle age with a slight predominance of male and show no specific presenting symptoms and characteristic roentgenologic manifestations (6). CT scan of the thorax is crucial in defining contiguous thoracic structures invasion (pleura, pericardium, vessels, chest wall), and it allows the determination of local extension. But diagnosis of primary lung sarcoma should be considered only when there is no evidence of a former treated soft tissue sarcoma, and if no sarcoma is detected by extensive clinical and diagnostic imaging studies. Most series reported a median survival near to 24 months, and a 3-year survival between 17% and 50% (7). Surgical treatment without lymph node resection is the therapy of choice in all patients and tumor size to be one of the most important prognostic factors (8). Radiotherapy con be used in case of incompletely resected tumors whereas adjuvant chemotherapy may be indicated, depending on the size and grade differentiation of the tumor (9). Leiomyosarcomas can be primary or secondary in the intestine. Secondary neoplastic involvement of the small bowel is more frequent than primary small bowel malignant neoplasms and it can occur by direct invasion, lymphatic, haematogenous or intraperitoneal metastasis (10). Tumour invasion of all or parts of the bowel wall leads to the most common symptoms like gastro-intestinal bleeding, abdominal pain, obstruction, perforation, malabsorption and peritonitis. Secondary small bowel involvement by leiomyosarcoma is extremely rare. Recent Behranwala’s report (11) evaluated abdominal metastasis from primary soft tissue sarcoma (STS) and described 19 patients, with an incidence 0.9%, 4 of whom (21.1%) affected by STS leiomyosarcoma with dismal survival after surgery. Other authors described small bowel metastasis from primary bone leiomyosarcoma (12) successfully treated with surgery and still alive after short-term follow up. There is a large agreement that small bowel metastases usually represent a poor prognostic indicator of lung carcinoma (13). Surgical treatment of GI metastasis is considered as a palliative treatment to prevent bowel obstruction or peritonitis (3).
To our knowledge this is the first report of small bowel metastases from a pulmonary leiomyosarcoma with good results in terms of disease-free survival after surgical resection.
In the management of lung sarcomas it is very important to differentiate primary pulmonary sarcoma by metastatic spread from an extra pulmonary sarcoma. It is therefore necessary to reconstruct carefully the clinical history, and to use appropriate investigations to address this possibility. In conclusion a prompt operative management is essential not only in distinguishing the lesion such as metastasis from a primary tumor or preventing potentially lethal abdominal complications but also in improving survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Miller DL, Allen MS. Rare pulmonary neoplasms. Mayo Clin Proc 1993;68:492-8. [PubMed]
- Berger A, Cellier C, Daniel C, et al. Small bowel metastases from primary carcinoma of the lung: clinical findings and outcome. Am J Gastroenterol 1999;94:1884-7. [PubMed]
- Lee PC, Lo C, Lin MT, et al. Role of surgical intervention in managing gastrointestinal metastases from lung cancer. World J Gastroenterol 2011;17:4314-20. [PubMed]
- Pollock RE, Karnell LH, Menck HR, et al. The National Cancer Data Base report on soft tissue sarcoma. Cancer 1996;78:2247-57. [PubMed]
- Arnold LM 3rd, Burman SD. O-Yurvati AH. Diagnosis and management of primary pulmonary leiomyosarcoma. J Am Osteopath Assoc 2010;110:244-6. [PubMed]
- Martini N, Hajdu SI, Beattie EJ Jr. Primary sarcoma of the lung. J Thorac Cardiovasc Surg 1971;61:33-8. [PubMed]
- Nascimento AG, Unni KK, Bernatz PE. Sarcomas of the lung. Mayo Clin Proc 1982;57:355-9. [PubMed]
- Janssen JP, Mulder JJ, Wagenaar SS, et al. Primary sarcoma of the lung: a clinical study with long-term follow-up. Ann Thorac Surg 1994;58:1151-5. [PubMed]
- Movsas B. Lung cancers. In: Pazdur R, Wagman LD, Camphausen KA. eds. Cancer Management: A Multidisciplinary Approach. 5th ed. Huntingdon, NY: PRR, Inc, 2001:487-506.
- Gill SS, Heuman DM, Mihas AA. Small intestinal neoplasms. J Clin Gastroenterol 2001;33:267-82. [PubMed]
- Behranwala KA, Roy P, Giblin V, et al. Intra-abdominal metastases from soft tissue sarcoma. J Surg Oncol 2004;87:116-20. [PubMed]
- Chiang KC, Yeh CN, Shih HN, et al. Lower gastrointestinal bleeding due to small bowel metastasis from leiomyosarcoma in the tibia. Chang Gung Med J 2006;29:430-4. [PubMed]
- Yang CJ, Hwang JJ, Kang WY, et al. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer 2006;54:319-23. [PubMed]


