Comparison of outcomes of open and minimally invasive esophagectomy in 183 patients with cancer
Introduction
It is estimated that about 16,980 individuals (13,450 men and 3,530 women) were diagnosed with esophageal cancer and 14,710 were reportedly died in 2011 in the United States of America (1). China is also one of the countries with the highest esophageal cancer risk in the world (2). Esophagectomy is a gold standard in the treatment of patients with localized esophageal carcinoma (3). However, esophagectomy for cancer is considered to be one of the most extensive and traumatic oncological surgeries, which is associated with marked perioperative morbidity and mortality (up to 60% and 14%, respectively) (4-6). Elderly patients and those with comorbid diseases may give up the operation because of this high mortality, which is mainly caused by pulmonary and cardiac complications.
In recent years, the success of minimally invasive surgery has revolutionized the management of the disorders in the gastrointestinal tract. In 2000, Luketich et al. (7) first reported the minimally invasive esophagectomy (MIE) and proved that the operation was as good as or better than the open esophagectomy (OE). Reducing the trauma related to surgical access directly results in lesser tissue injury, blood loss, postoperative pain, analgesic requirements, and impairment of respiratory and cardiac function. This potentially allows a more rapid recovery and helps to return to normal health-related quality of life (3,8).
Only few randomized trials or comparative studies with large number of patients have been reported on the outcomes of these procedures. Most comparative studies showed clinical advantages such as shorter operation times, fewer blood loss, shorter intensive care unit (ICU) and hospital stays, as well as a similar survival (9-11). One of the problems arising when comparing MIE and OE is the effect of selection bias on nonrandomized studies. The aim of the present study was to compare the postoperative outcomes and survival of patients with esophageal cancer who underwent thoracoscopic and laparoscopic esophagectomy (TLE) or OE.
Patients and methods
Patients and clinical data
Clinical and surgical data of 183 patients with esophageal cancer, who underwent TLE or 3-field OE between February 2011 and December 2013, were included in this retrospective study. Diagnosis of all the patients was established by esophagoscopy and biopsies; computed tomography and endoscopic ultrasound scans were used to evaluate the resectability of tumor. Resectable esophageal cancer was defined and patients were included in the study per the following eligibity criteria: cT1-3, N0-1, M0; esophageal cancer involving the gastric cardia was excluded; Eastern Cooperative Oncology Group performance scores of 0-2; tolerable pulmonary function under double lung ventilation for thoracotomy operation; normal functions of vital organs; normal blood detection; no previous thoracic, hiatal, or bariatric surgery; and no history of preoperative neoadjuvant chemotherapy and radiotherapy. All operations performed by a group of surgeons, who worked together for several years with experience in OE and TLE with at least 10 MIEs, were considered. The other criterion of operation was as follows: the gastric tube, as a substitute material of esophagus, was used to reconstruct the upper digestive tract through the mediastinal esophageal bed, and gastroesphageal track was anastomosed in the left neck by hands.
Operative technique
The 3-field OE was performed through an upper midline abdominal incision, right thoracotomy, and left neck incision. The procedure was described in detail by McKeown (12). The surgeons learned the technology and were trained in the TLE procedure as a modification of the original operation described by Luketich’s (8) and Dr. Tan’s (13). With the control and skill of the technique, a lot of own characteristics were also added to the knowledge on technology to perform surgeries.
TLE with cervical anastomosis
Patients were intubated with a double-lumen endotracheal tube and placed in left lateral semi-prone position. The surgeon stood on the right and the assistant on the left. Four thoracoscopic ports were used. Artificial pneumothorax was established by carbon dioxide (CO2) (pressure: 12 mmHg and flow velocity: 20 L/min), thus providing downward traction on the diaphragm and allowing good exposure of the distal esophagus.
Thoracoscopic mobilization of the esophagus and systematic lymph node dissection
After mobilizing the inferior pulmonary ligament, the mediastinal pleura overlying the esophagus were divided up to the level of azygos vein by an electrical coagulation hook. After double clipping of the azygos vein by Ham-o-lok at each side, the vessel was divided by ultrasonic coagulation shears. Circumferential mobilization of the entire esophagus was performed up to the thoracic top and down to the plane of diaphragm, with removal of paraesophageal and subcarinal lymph nodes. Bilateral recurrent laryngeal nerve lymph nodes were cleaned by sharp dissections. A chest tube and mediastinal drainage tube were placed. The mediastinal drainage tube was placed along the esophageal bed until to the top of chest; hence, the procedure in the thoracic parts was completed. The lung was allowed to inflate; any air leaks from the trachea, proximal bronchus, and re-expanded lung were carefully observed.
Ligation and transection of the esophagus
The patient was turned to supine position. An incision of 4 cm was cut on the left cervical skin first. The cervical esophagus was exposed directly through a left anterior sternomastoid incision and dissection to the level of the cricoid prior to deliver the gastric conduit. The cervical esophagus was dissected with the fingers, ligated it with two sutures, and transected the esophagus by electrical coagulation knife. Two sutures were tied with another suture to extend the length for pulling out the stomach and esophagus easily.
Laparoscopic mobilization of the stomach and abdominal lymph node dissection
The patient was kept in a supine position, surgeon stood on the patient’s right, and first assistant was standing on the left of the patient. Artificial CO2 pneumoperitoneum was established (pressure: 15 mmHg and flow velocity: 40 L/min). The laparoscopic ports were little different from Luketich’s. A 10 mm camera port was created at the right paraumbilical line with a height of 2 cm. The main operating hole (10 mm) was located at the left paraumbilical line with a height of 2 cm, and the second operating hole (5 mm) was located under the costal margin of the left mid-clavicular line. One of assistant’s operation holes (10 mm) was created below the xiphoid process and another (5 mm) was on umbilical level of anterior axillary line. The stomach was mobilized by dividing the short gastric vessels using the ultrasonic coagulating shears. The gastrocolic omentum was carefully divided to preserve the right gastroepiploic arcade. After double clipping of the left gastric artery by Ham-o-lok at each side, the vessel was divided by ultrasonic coagulation shears. Lymph nodes and fat tissues along the left gastric vessels, celiac axis, common hepatic artery, and splenic artery were dissected.
Gastric tube construction and cervical anastomosis
The xiphoid port was extended to 5 cm, along the abdominal midline. The specimens from the mini-incision were then dissociated out. A gastric tube of 5 to 6 cm in diameter was constructed along the great curvature by linear cutter or hand sewing. Then, the specimen and proximal gastric cardia were moved. The gastric conduit was pulled up to the neck under laparoscopic guidance, and esophagogastric anastomoses was performed with the three-leaf clipper-assisted manual layered anastomosis technique (14). A nasojejunal feeding tube was guided into the jejunum, and a gastrointestinal decompress tube was placed through nasal cavity. The surgery was finished after closure of the cervical and abdominal incisions.
The treatment principle in perioperative period was identical between both groups. The patients were transferred to the general ward after awakening from anesthesia, and the patients were transferred to ICU if there were breathing complications. All the postoperative patients were instructed to take a deep breath and assisted cough, and they were given liquid food through a nasal feeding after gastrointestinal exhaust. Gastrointestinal decompression was implemented until day 5-6 after surgery, and the patients were given liquid diet which then was converted to a semi-liquid diet from day 7 after the surgery. The patients were discharged from hospital when they could eat semi-liquid food without any trouble and walk without any discomfort.
Postoperative follow-up
The patients were regularly followed up mainly by outpatient service and telephone after surgery. The outpatient follow-up was performed once in the first month after hospital discharge, once in every 3 months till the first 2 years, and thereafter once every 6 months. Death and lost to follow-up were defined as events and were recorded.
Statistical analysis
Statistical analysis was performed by Statistical Package for Social Sciences (SPSS version 17.0). Comparison of data between TLE and OE was done using the Student’s t-test for continuous data and the chi-square tests for categorical data. Survival was calculated with the Kaplan-Meier method. A value of P<0.05 was considered to be statistically significant.
Results
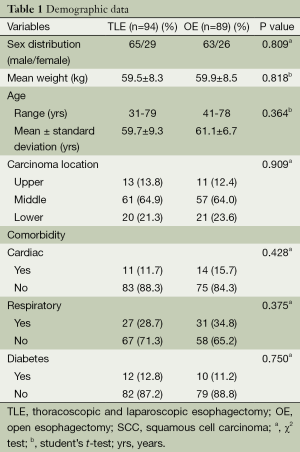
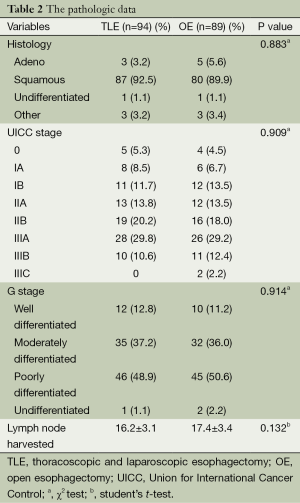
This retrospective study included a total of 183 patients who received either OE or MIE. There were no significant differences in demographic and pathologic characteristics of patients (Tables 1,2).
Full table
Full table
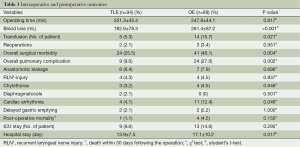
The intraoperative and postoperative outcomes are shown in Table 3. The TLE group had significantly less blood loss, and fewer patients underwent blood transfusion. The total dissection number of lymph nodes in these two groups was 1,527 vs. 1,548 (mean: 16.2 vs. 17.4), respectively. The mean number of positive lymph node is 0.67% vs. 1.15%, respectively (P>0.05). Nine (9.6%) patients of TLE group and 13 (14.6%) patients of OE group required to send to ICU as a consequence of complications. Mean hospital stay was significantly shorter in TLE group than OE group.
Full table
The overall surgical morbidity in the TLE group was significantly lower compared with the OE group. There was a significantly lower rate of pulmonary complications and cardiac arrhythmia in the TLE group. There was no statistical significance with the disparity of anastomotic leak and recurrent laryngeal nerve injury between the two groups. Other complications like chylothorax and diaphragmatocele were similar between the two groups. Unfortunately, one patient in TLE group and four patients in OE group died within 30 days. The patient died after TLE was due to acute gastrointestinal bleeding, which was also the reason for one of the four patients died in the OE group. Other patients died in the OE group because of pulmonary complications (n=1) and chest infection as anastomotic leak (n=2).
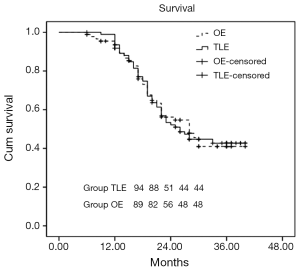
The follow-up of two groups ranged from 6 to 40 months. The Kaplan-Meier survival curve is shown in Figure 1. Four patients in the OE group and three in the TLE group were lost to follow-up. Median follow-up was 28 months (standard error 2.4; 95% CI, 23.3-32.7). The log-rank test showed no difference between the two groups (P=0.993). Median survival for patients in OE was 28 months (standard error 3.2; 95% CI, 21.8-34.2) compared with 26 months (standard error 2.6; 95% CI, 20.8-31.2) in the TLE group.
Discussion
The present retrospective study has shown that TLE and OE are both safe and feasible for esophagogastric cancer with comparable morbidity, surgical outcomes, and overall survival. These findings are consistent with other reported studies (8-11). In the present study, the TLE procedure resulted in similar or potentially better outcomes, although the survival was not different between the TLE and OE groups.
The advantages of position and incisions in operation had been reported in previous literatures (15). With the continuous search of optimal operation technology, the cutting of esophagus in the neck was replaced as it was not only convenient for operation, but it could also decrease the cost and chance of contamination and could ensure the integrity of tumor. As the chest incision was only about 1 cm in the TLE group, it could avoid the shortcomings of traditional OE such as large incision, ribs distraction, and destruction of the abdominal wall integrity. The blood loss was significantly lower in the TLE group, which was consistent with the literature (3,7,8). Due to the lower blood loss, only fewer patients required blood transfusion. Postoperative mortality and ICU stay did not differ significantly; however, the overall surgical morbidity was significantly lower in TLE, suggesting TLE as a safer procedure with acceptable complication rates.
The most common complications after the surgery mainly include anastomotic fistula, pulmonary complications, arrhythmia, delayed gastric emptying, chylous leakage, and recurrent laryngeal nerve injury. Currently, it is still controversial that the thoracoabdominal endoscopic esophageal resection can reduce the postoperative pulmonary complications. Smithers et al. and some others researches (16,17) suggested that the thoracoabdominal endoscopic esophageal resection did not reduce the incidence of postoperative pulmonary complications, but it even increased the incidence of postoperative pulmonary complications. However, some studies have shown that the MIE can significantly reduce postoperative pulmonary complications in patients (8,18-20). Recently, Sihag et al. (21) have found that MIE is the only relevant factor in significantly reducing pulmonary complications. In the present study, the incidence of complication in the TLE group was significantly lower compared with the OE group, which was mainly attributed to the obvious decrease in the complications of heart and lung.
Lymph nodes around the recurrent laryngeal nerve are the positions where transfer of esophageal cancer could easily occur, leading to worse disease-specific survival (22). In addition, the damage of recurrent laryngeal nerve will lead to a series of complications and poor prognosis (23,24). Hence, it is important to pay attention to the protection of the recurrent laryngeal nerve at lymph node dissection, while carefully identifying and preventing accidental injury and taking care of the injuries caused by the ultrasonic scalpel heat conduction. There is a higher difficulty for cleaning the left recurrent laryngeal nerve lymph node using a thoracoscope. In addition to preventing accidental injury of the recurrent laryngeal nerve, the membrane departments of trachea also need much attention. In the present study, there was no significant difference between groups with respect to recurrent laryngeal nerve injury, suggesting TLE as a safer procedure.
With the development of surgical techniques, the occurrence of postoperative anastomotic fistula is significantly reduced. In the present study, there was no significant difference in the incidence of anastomotic leakage, chylothorax, diaphragmatocele, and delayed gastric emptying. However, pleural mediastinal infection caused by the anastomotic fistula is also very dangerous in postoperative patients. At present, the key treatment of this condition is drainage; therefore, mediastinal drainage tube should be conventionally placed before closing the chest in patients undergoing surgery for esophageal cancer (25). Mediastinal drainage tube is placed in the mediastinal esophageal bed, while the upper end directly gets to the chest top and the lower end is fixed in the seventh intercostal space. The vacuum extractor plays a role in drainage, but it can also act as a role of wash pipe, if necessary. Therefore, if the drainage volume is not large after the surgery, the chest tube can be removed on 2-3 days after surgery. Thus, the pain of patient can be reduced as soon as possible. The patients should be encouraged to get out of bed early to accelerate the functional recovery. Nasojejunal tube was also used as opposed to a transcutaneous jejunal tube to reduce the trauma as much as possible in both groups. In the present study, the hospital stay of TLE group was 3 days shorter than OE group. This finding shows that TLE has an obvious advantage than traditional methods.
Rough comparisons with recent reports on OE suggest that reduced perioperative morbidities, especially cardiopulmonary complications and blood loss, plus a shorter postoperative hospital stay are areas in which MIE might prove to be superior. However, surgeons are more concerned about the possibility to enhance long-term survival after the surgery. The patients were followed up for 6-40 months in this study, and the median follow-up was 28.0±2.4 months (95% Cl, 23.3-32.7); in which, the median follow-up in the TLE and OE groups were 26.0±2.6 months (95% Cl, 20.8-31.2) and 28.0±3.2 months (95% Cl, 21.8-34.2), respectively. The survival rates in the both the groups were 42.7% and 41% (Log-rank test: P=0.993), respectively (Figure 1). There was no significant difference in survival time, which was consistent with published literature (3,9,11).
Conclusions
In summary, the thoracoabdominal endoscopic esophageal resection is not only technically feasible and safe, but it can also achieve the same radical effect of tumor as the conventional three incisions surgery. The more important is that the surgical method can significantly reduce the bleeding amount, reduce the incidence of perioperative cardiopulmonary complication, and reduce postoperative hospital stay. Although it has not fount to extend the time of the long-term survival in the patients with esophageal cancer, the endoscopic technology still has a potential advantage and is a treatment method worthy to be popularized.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- U.S. National Institutes of Health. SEER Stat Fact Sheets: Esophagus. Available online: http://seer.cancer.gov/statfacts/html/esoph.html
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Smithers BM, Gotley DC, Martin I, et al. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg 2007;245:232-40. [PubMed]
- McCulloch P, Ward J, Tekkis PP, et al. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ 2003;327:1192-7. [PubMed]
- Law S, Wong KH, Kwok KF, et al. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg 2004;240:791-800. [PubMed]
- Biere SS, Maas KW, Bonavina L, et al. Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial). BMC Surg 2011;11:2. [PubMed]
- Luketich JD, Schauer PR, Christie NA, et al. Minimally invasive esophagectomy. Ann Thorac Surg 2000;70:906-11; discussion 911-2. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [PubMed]
- Zingg U, McQuinn A, DiValentino D, et al. Minimally invasive versus open esophagectomy for patients with esophageal cancer. Ann Thorac Surg 2009;87:911-9. [PubMed]
- Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009;64:135-46. [PubMed]
- Parameswaran R, Veeramootoo D, Krishnadas R, et al. Comparative experience of open and minimally invasive esophagogastric resection. World J Surg 2009;33:1868-75. [PubMed]
- McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus. Br J Surg 1976;63:259-62. [PubMed]
- Wang H, Feng M, Tan L, et al. Comparison of the short-term quality of life in patients with esophageal cancer after subtotal esophagectomy via video-assisted thoracoscopic or open surgery. Dis Esophagus 2010;23:408-14. [PubMed]
- Zhu ZJ, Zhao YF, Chen LQ, et al. Clinical application of layered anastomosis during esophagogastrostomy. World J Surg 2008;32:583-8. [PubMed]
- Gao Y, Wang Y, Chen L, et al. Comparison of open three-field and minimally-invasive esophagectomy for esophageal cancer. Interact Cardiovasc Thorac Surg 2011;12:366-9. [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [PubMed]
- Smithers BM, Gotley DC, Martin I, et al. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg 2007;245:232-40. [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg 2006;203:7-16. [PubMed]
- Dapri G, Himpens J, Cadière GB. Minimally invasive esophagectomy for cancer: laparoscopic transhiatal procedure or thoracoscopy in prone position followed by laparoscopy? Surg Endosc 2008;22:1060-9. [PubMed]
- Zingg U, Smithers BM, Gotley DC, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol 2011;18:1460-8. [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [PubMed]
- Greenstein AJ, Litle VR, Swanson SJ, et al. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg 2008;206:239-46. [PubMed]
- Hulscher JB, van Sandick JW, Devriese PP, et al. Vocal cord paralysis after subtotal oesophagectomy. Br J Surg 1999;86:1583-7. [PubMed]
- Safranek PM, Cubitt J, Booth MI, et al. Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg 2010;97:1845-53. [PubMed]
- Qin J, Li Y, Zhang R, et al. Treatment of esophagogastric anastomotic leak with perianastomotic drain. J Thorac Oncol 2010;5:251-3. [PubMed]


