An analysis of and new risk factors for reexpansion pulmonary edema following spontaneous pneumothorax
Introduction
Reexpansion pulmonary edema (RPE) is a potentially life-threatening complication that can occur after rapid lung reexpansion following the treatment of pneumothorax or pleural effusion. RPE was first described by Pinault in 1853 as a complication of thoracentesis (1) and in 1959, Carlson et al. reported that RPE occurred after treatment of a pneumothorax (2). Since then, there have been many reports regarding RPE. Although the incidence of RPE varies from 0.9% to 29.8% (3-5), the mortality rate associated with RPE can be as high as 20% (6,7). Therefore, early recognition and fast symptom-orientated treatment are necessary for a good outcome. However, RPE usually appears unexpectedly, although several studies about RPE, including investigations of its clinical features, treatment or prevention, have been reported. RPE can occur even when the collapse lasts for less than 3 days (4), although in the comprehensive review of RPE by Mahfood and colleagues (6), 83% of the cases had experienced periods of lung collapse lasting 3 or more days. Ultimately, the risk factors for RPE remain unclear. In this study, we wanted to analyze the clinical characteristics and risk factors of RPE by retrospectively assessing the clinical records of patients with spontaneous pneumothorax who were treated by thoracostomy.
Patients and methods
Patients
We retrospectively reviewed the clinical records of all patients hospitalized in our surgical department after they had undergone drainage for the treatment of spontaneous pneumothorax in our institution between January 2007 and December 2012. Patients with a known history of underlying lung disease (mainly chronic obstructive pulmonary disease) were excluded. All patients underwent erect posteroanterior chest radiography to verify the presence of a spontaneous pneumothorax. Subsequent radiographs and chest computed tomography (CT) scans were obtained within 24 hours of thoracostomy to confirm the lung reexpansion, as well as the presence of complications such as tube malposition, RPE or the presence of bullae. The methods used for the lung expansion included high negative suction with needle aspiration, a chest tube with an underwater seal or suction.
Diagnostic criteria for RPE
A diagnosis of RPE was made on a radiographic basis. The radiographic criteria included a chest radiograph or CT scan with a new finding of focal ground-glass opacity with a vascular distribution (8).
Classification of the pneumothorax sizes
The pneumothorax size was measured according to the method described by Collins et al. (9). This method used CT volumetry to derive a formula based on measurements of the interpleural distances on a chest radiograph to estimate the pneumothorax size. The formula requires measurements of the interpleural distance at the apex (A) and the lateral wall at the mid-point of the upper and lower halves of the collapsed lung (B and C).
Estimated pneumothorax size (%) =4.2+ [4.7 + (A + B + C)]
We classified the patients based on the size of their pneumothorax into the following four groups: small, medium, large and tension (5). A small pneumothorax was defined as a pneumothorax that was localized to the apex of the lung on chest radiography. A medium pneumothorax was defined as a pneumothorax that extended beyond one-third of the width of a hemithorax. A large pneumothorax was defined as a pneumothorax leading to complete or nearly complete collapse of the lung parenchyma. A tension pneumothorax was defined as a pneumothorax associated with depression of the diaphragm or a shift of the mediastinum and trachea away from the collapsed lung.
Classification of the chest tube sizes
We also classified the patients based on chest tube sizes used for drainage into the following three groups: small (≤14 Fr), medium (16 to 22 Fr) and large (24 to 36 Fr) (10).
Classification of pleural effusion
In general, pleural effusion can occur coincident with pneumothorax, although it is usually quite small.
The determination of the presence of pleural effusion was made on erect posteroanterior chest radiographs which were performed to confirm the presence of a spontaneous pneumothorax. The patients were also classified into three sub-groups based on the appearance of effusion (11-13):
- No effusion or very small effusion: a sharp costophrenic angle;
- Small: blunting of the costophrenic angle;
- Moderate: the partial outline of the diaphragm on the affected side is lost with the meniscus sign;
- Large: the entire outline of the diaphragm on the affected side is lost.
Statistical methods
The continuous data are presented as means with SDs, and were compared with the independent sample t-test or Mann-Whitney U-test, as appropriate. Nominal data are presented as the percentages of the frequency of occurrence and were compared with a χ2 or Fischer exact test, as appropriate. A univariate analysis of the lesions characteristic was used to calculate the risk and odds ratios (OR) with confidence intervals (CI). A multivariate logistic regression analysis was then performed. Values of P≤0.05 were considered to be statistically significant.
Results
Between January 2007 and December 2012, 40 patients were diagnosed with a spontaneous pneumothorax and treated with tube thoracostomy. The patients ranged in age from 15 to 64 years old (mean, 26.6±12.3 years old) and there were 32 males and 8 females (Table 1). The mean duration of symptoms was 5.1±8.4 days. The pneumothorax was located on the right side in 21 patients, on the left side in 18 and bilaterally in one patient. With regard to the pneumothorax size, 18 patients were classified in the small group, 9 patients were classified as having a medium pneumothorax, 7 were classified as large and 6 patients were classified as having a tension pneumothorax. Of the chest tubes inserted for treatment, 9 were small, 23 were medium sizes and 8 were large. Pleural effusion was not detected in 18 patients, was small in 17 patients, moderate in 5 patients (Figure 1), and there were no patients with large effusion.
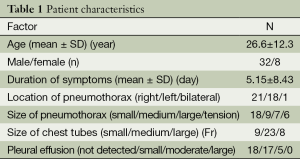
Full table
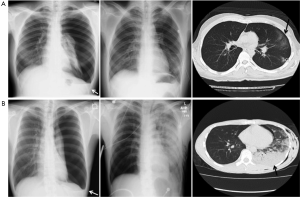
RPE developed in 13 (32.5%) of the 40 patients with a spontaneous pneumothorax that was treated by thoracostomy. Four of these patients developed a mild cough and dyspnea, but no patients developed respiratory failure or death, and nine patients were asymptomatic and did not require specific therapy. The CT image findings of RPE were limited to one pulmonary lobe in 8 of 13 patients, two lobes in three and three lobes in two patients. No patients develop RPE in the contralateral lung.
The factors that could have contributed to the RPE were evaluated from a comparison of patients with and without RPE. These factors are shown in Table 2. These were followed by a multivariate analysis (Table 3). The duration of symptoms (OR, 1.004; 95% CI, 1.000-1.008) and size of the pneumothorax (OR, 0.996; 95% CI, 0.980-1.011) were not significant risk factors for RPE, but the pleural effusion was found to be a risk factor for RPE (OR, 1.557; 95% CI, 1.290-1.880).
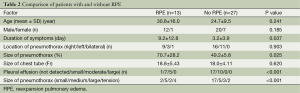
Full table
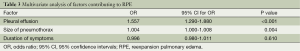
Full table
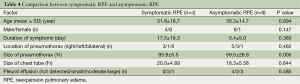
A further analysis was performed to evaluate the risk factors contributing to symptomatic RPE based on a comparison of patients with and without symptoms of RPE (Table 4). The size of the pneumothorax was significantly larger in patients with symptomatic RPE than in those with asymptomatic RPE [(95.9±5.5)% vs. (59.5±26.9)%; P=0.005], although there were no significant differences in the duration of symptoms, size of the chest tube or volume of pleural effusion between patients with symptomatic RPE and asymptomatic RPE.
Full table
Discussion
In our study, the incidence of RPE was much higher (32.5%) than in the series of RPE published to date (3-5), which was probably due to our CT-based diagnosis of RPE according to the criteria as described above, whereas previous studies used a chest radiographic diagnosis of RPE. Indeed, it may be not cost effective to proceed with CT immediately for the diagnosis of pneumothorax. However, CT was needed in order to evaluate the bullas or blebs for treatment options including surgery in our institution. CT imaging is apparently more sensitive than plain radiography for diagnosing RPE (2). As a result, even small and asymptomatic cases of RPE could be identified in our study. In general, the symptoms of RPE include a new cough, worsening dyspnea, hypoxia, tachypnea, or hemodynamic instability (5,14). The best treatment is thought to be supportive, mainly consisting of the administration of supplemental oxygen and morphine if needed (15). The use of diuretics or steroids may also be effective (16). In our study, there were transient symptoms, such as a mild cough and dyspnea, in some patients, and no respiratory failure or death in any of the patients with symptomatic RPE, and no treatment was necessary for any of the patients with asymptomatic RPE, although RPE had been thought to have a high mortality rate in previous studies (6,7). These findings indicate that RPE is a more common, transient and benign phenomenon than was previously thought.
The radiographic diagnosis of asymptomatic RPE may be clinically insignificant, because it does not require any specific therapy (17). Therefore, it is important to identify risk factors for symptomatic RPE. Several risk factors for RPE have been proposed, including the duration of symptoms (5,18,19), a larger size of the pneumothorax (4,5,19), younger age (4) and a rapid rate of reexpansion (20). However, the duration of symptoms and size of the pneumothorax were not significant risk factors for RPE in our study. On the other hand, we found that the presence of pleural effusion coincident with pneumothorax was associated with the development of RPE. To the best of our knowledge, no previous studies have investigated this association.
In our present study, the size of the pneumothorax was also significantly larger in patients with symptomatic RPE than in those with asymptomatic RPE (6). Mahfood et al. reported that 64% of patients exhibited symptoms within 1 hour after reexpansion, and in all cases, the onset occurred within 24 hours (6). Accordingly, physicians should pay particular attention to the clinical course of the patient for 24 hours when the post-procedure images show findings of RPE following the treatment of a large pneumothorax which is coincident with moderate pleural effusion, even if the patient is asymptomatic. Although the exact mechanism(s) underlying the development of RPE is still not completely understood, the possible pathogenic events leading to RPE may include pulmonary vascular injury and an increase in capillary permeability (17,21). The development of pleural effusion which is coincident with the pneumothorax may also be caused by these mechanisms, because the patients with a known history of underlying lung disease were excluded and pleural effusion was associated with RPE in our study.
It may be difficult to prevent the development of RPE even if physicians can predict it based on the risk factors, because the thoracostomy procedure itself, which is an effective method to prevent the development of RPE, tends to be both a complex and difficult procedure to perform. According to previous guidelines (13,22), it has been recommended that the collapsed lung should be reexpanded by using a small-bore catheter (14 Fr) or chest tube (16 to 22 Fr) in clinically stable patients, or by using a larger chest tube (24 to 28 Fr) in unstable patients, and that suction should not be routinely employed. Although the procedure was undertaken in compliance with the guidelines in our cases, we could not prevent RPE. Future studies will hopefully identify a new procedure or type of postoperative care that can be used to prevent RPE.
In conclusion, the incidence of RPE appears to be higher than has been reported in previous studies. Furthermore, it often remains asymptomatic. Of note, the size of the pneumothorax was significantly greater in symptomatic RPE than in asymptomatic RPE. Our findings therefore suggest that the presence of pleural effusion coincidentally with pneumothorax may be a new risk factor for RPE.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Pinault HA. Considérations cliniques sur la thoracentèse. Paris: Impr. Rignoux, 1853.
- Carlson RI, Classen KL, Gollan F, et al. Pulmonary edema following the rapid reexpansion of a totally collapsed lung due to a pneumothorax: a clinical and experimental study. Surg Forum 1958;9:367-71. [PubMed]
- Rozenman J, Yellin A, Simansky DA, et al. Re-expansion pulmonary oedema following spontaneous pneumothorax. Respir Med 1996;90:235-8. [PubMed]
- Matsuura Y, Nomimura T, Murakami H, et al. Clinical analysis of reexpansion pulmonary edema. Chest 1991;100:1562-6. [PubMed]
- Kim YK, Kim H, Lee CC, et al. New classification and clinical characteristics of reexpansion pulmonary edema after treatment of spontaneous pneumothorax. Am J Emerg Med 2009;27:961-7. [PubMed]
- Mahfood S, Hix WR, Aaron BL, et al. Reexpansion pulmonary edema. Ann Thorac Surg 1988;45:340-5. [PubMed]
- Trachiotis GD, Vricella LA, Aaron BL, et al. As originally published in 1988: Reexpansion pulmonary edema. Updated in 1997. Ann Thorac Surg 1997;63:1206-7. [PubMed]
- Gleeson T, Thiessen R, Müller N. Reexpansion pulmonary edema: computed tomography findings in 22 patients. J Thorac Imaging 2011;26:36-41. [PubMed]
- Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol 1995;165:1127-30. [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [PubMed]
- Burgener FA, Kormano M, Pudas T. The Same But Better: Differential diagnosis in conventional radiology. Thieme Medical Publishers 2008; 80:174-175.
- Broaddus VC, Light RW. Chapter 73 – Pleural Effusion. In: Mason RJ, Broaddus VC, Martin TR, et al. eds. Murray and Nadel’s Textbook of Respiratory Medicine, 5th ed. Philadelphia: Saunders, 2010.
- Woodring JH. Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR Am J Roentgenol 1984;142:59-64. [PubMed]
- Feller-Kopman D, Walkey A, Berkowitz D, et al. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest 2006;129:1556-60. [PubMed]
- Rozenman J, Yellin A, Simansky DA, et al. Re-expansion pulmonary oedema following spontaneous pneumothorax. Respir Med 1996;90:235-8. [PubMed]
- Sohara Y. Reexpansion pulmonary edema. Ann Thorac Cardiovasc Surg 2008;14:205-9. [PubMed]
- Feller-Kopman D, Berkowitz D, Boiselle P, et al. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007;84:1656-61. [PubMed]
- Murphy K, Tomlanovich MC. Unilateral pulmonary edema after drainage of a spontaneous pneumothorax: case report and review of the world literature. J Emerg Med 1983;1:29-36. [PubMed]
- Tan HC, Mak KH, Johan A, et al. Cardiac output increases prior to development of pulmonary edema after re-expansion of spontaneous pneumothorax. Respir Med 2002;96:461-5. [PubMed]
- Kernodle DS, DiRaimondo CR, Fulkerson WJ. Reexpansion pulmonary edema after pneumothorax. South Med J 1984;77:318-22. [PubMed]
- Sherman SC. Reexpansion pulmonary edema: a case report and review of the current literature. J Emerg Med 2003;24:23-7. [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [PubMed]


