Is video-assisted thoracic surgery lobectomy in benign disease practical and effective?
Introduction
Lobectomy can be performed in both malignant and benign disease. It can be applied in localized or medically-resistant pulmonary tuberculosis or in pulmonary nodules to confirm histologic features (1-4). It is also necessary in the treatment of pulmonary sequestration or congenital cystic adenomatoid malformation (5-8).
Video-assisted thoracic surgery (VATS) had been widely accepted because of its low complication rate, tolerable postoperative pain and early recovery of pulmonary function (9-12). However it still remains controversial whether it is recommendable in benign diseases or not. Because benign disease tends to have pulmonary adhesion, lymph node enlargement, and neovascularization (2,3), these inhibit successful VATS procedure and sometimes require conversion to thoracotomy (2,3). However, little is known about its ideal role because of the paucity of thorough studies of VATS lobectomy for benign disease compared to for malignant disease (11,13).
Therefore, the aim of this study was to analyze the surgical outcomes of VATS lobectomy for benign pulmonary disease and to gain insight into the most suitable surgical conditions for this approach.
Methods
Patient enrollment
From January 2004 to December 2009 a total of 3,476 (1,067 by VATS, 2,409 by thoracotomy) lobectomies were performed at a university-based tertiary care hospital in Seoul, Korea. Inclusion criteria were (I) patients who had benign disease treated by VATS lobectomy; and (II) thoracotomy conversion cases initially approached by VATS lobectomy. Thoracotomy conversion was defined as a procedure that started with VATS for lobectomy, but was ultimately converted to thoracotomy for any reason. The exclusion criteria were as follows: (I) not a lobectomy; (II) lobectomy undergone by thoracotomy without any attempt at VATS; or (II) a VATS lobectomy for malignant disease. This study was reviewed and approved by the Institutional Review Board of Samsung Medical Center.
Technique
A 15-mm trocar for the 10-mm 30-degree thoracoscope was placed through the sixth intercostal space. A 4- to 5-cm utility incision was made through the fourth or fifth intercostal space in the anterior axillary line without rib spreading. Subsequently, an additional 5- to 10-mm trocar was placed through the sixth or seventh intercostal space in the posterior scapular line. Individual dissection of pulmonary vessels and bronchi was attempted, and they were divided by endoscopic staplers (or surgical clips for vessels). Thoracotomy conversion was performed by extending the utility incision or by connecting two separate VATS ports.
Analysis
We retrospectively analyzed the electronic medical records to analyze gender, age, and surgical information including diagnosis, pleural adhesion, operation time, and blood loss and thoracotomy conversion. Then, we compared the data according to the disease entity, defined as follows: (I) infection group—patients who were diagnosed as having any kind of infection; pulmonary tuberculosis, non-tuberculous mycobacteria, and fungus; (II) non-infection group—congenital disease including congenital cystic adenomatoid malformation or pulmonary sequestration, benign nodule, or bronchiectasis. Primary outcomes were the thoracotomy conversion rate, period of thoracic drainage, length of hospital stay, and complications. Complications were divided into three categories: (I) none-no complications; (II) fatal-acute lung injury, acute respiratory distress syndrome, and bronchopleural fistula; (III) mild-others not including (I) or (II). Subsequently the results were compared between the two groups and follow-up results were also analyzed.
Descriptive statistics were used to describe patient characteristics and outcomes. Continuous variables were manifested as means and standard deviations and categorical variables were presented as numbers and proportions. Student’s t-test and the chi-square or Fisher’s exact tests were used to compare the continuous and categorical variables, respectively. P values less than 0.05 were considered statistically significant. SPSS 12.0K Windows software was used for the analyses.
Results
Basic demographics and surgical information
VATS lobectomy for benign disease was performed in 163 patients. There were 385 lobectomies for benign disease within the study period. Therefore 42% (163 of 385) lobectomies for the benign disease were performed by VATS. Of these, 60% (n=99) were women, and the mean age was 44 years old. Pleural adhesion was seen in half of the patients. Mean operation time and blood loss were 160 minutes and 326 mL, respectively. Thoracotomy conversion was necessary in 6 (4%) patients, with difficulty in hilar dissection being the most common reason (n=4).
There were 68 cases with infection (pulmonary tuberculosis 31, fungal infection 37) and non-infection was recognized in 95 (congenital disease 25, bronchiectasis 29, and benign nodule 41). Age and gender were similar between them. Pleural adhesion was more frequent in the infection group, but the operation time and blood loss was slightly higher in the non-infection group. However, there was no statistically significant difference (P value =0.40, 0.92, 0.63). Thoracotomy conversion was necessary in 2 (3%) of the infection group, and 4 (4%) in the group without infection. Detailed information was written in Table 1.
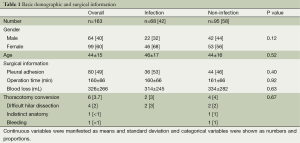
Full table
Surgical outcomes
The thoracotomy conversion rate was similar between the two groups (P value =0.67). Mean duration of a thoracic drainage and length of the hospital stay were about 5 and 7 days, respectively (P value =0.18, 0.25). Complications developed in 35 patients but 5 were fatal (3%). They were more in the infection group but there statistical difference was not seen (P value =0.50). On follow up 161 patients were cured (99%) and recurrence was seen in 2 (1%) patients. All recurrences developed in the infection group (pulmonary tuberculosis) and treated by medication. Detailed information was written in Table 2.
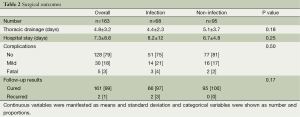
Full table
Comment
We set out to determine the role of VATS lobectomy in benign diseases. While there are many reports about VATS lobectomy for malignancy (9,10,13-15) there is a paucity of reports on VATS lobectomy for benign disease (2,3). Because benign diseases requiring lobectomy tend to have infection or inflammation, some surgeons choose open thoracotomy rather than VATS. The present study clearly demonstrates that VATS lobectomy is a practical and viable option, and is effective in selected benign disease cases. The thoracotomy conversion rate was low (3.7%) and the cure rate was high (98%). Moreover clinical outcomes were similar regardless of the presence of infection or not. Although the slightly long operation time and hospital stay was minor drawbacks, it is certain that many lobectomies for benign disease can be performed successfully by VATS.
However, careful attention is required in case selection. Half of the patients showed pleural adhesion and six patients required thoracotomy conversion. Moreover, the operation time and blood loss was about 160 minutes and 320 mL, respectively. Considering that these results were from a high-volume hospital, it is possible that these statistics could be poorer in other institutions. Moreover, the surgery was performed in selected cases. VATS was not attempted if severe pleural adhesion was suggested by preoperative chest computed tomography (CT) scan findings and thoracotomy was the first approach of choice if tight hilar adhesion was found on initial thoracoscopic exploration. In other words, VATS lobectomy for benign disease is feasible in selected cases but the choice can be difficult.
This study has some limitations. First, since it was a long-term study, the indication of VATS was extended during the study period. Therefore in some candidates VATS lobectomy were excluded in the early study period. Moreover technical developments could have enhanced results to some extent later in the study period. Second, multiple disease entities were included. For example, pulmonary tuberculosis and fungal infection may be similar in terms of being infectious diseases but clinicopathologic features and treatment strategies can be different. It is therefore difficult to draw categorical conclusions from this study. However, the aim of this study was not a mere comparison of the surgical results for different benign diseases, but to show the practicality and effectiveness of VATS lobectomy for benign disease. We believe this study achieved this goal, based on a substantial number of patients in a single institution.
Conclusions
In conclusion, VATS lobectomy for benign disease is feasible and effective in selected cases, regardless of the presence of infection. However, there various technical obstacles may be present during the procedure, therefore, careful patient selection and meticulous operation are both required.
Acknowledgements
The authors are indebted to J. Patrick Barron, Professor Emeritus of Tokyo Medical University, and Adjunct Professor, Seoul National University Bundang Hospital, for his pro bono editing of this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Petersen RH, Hansen HJ, Dirksen A, et al. Lung cancer screening and video-assisted thoracic surgery. J Thorac Oncol 2012;7:1026-31. [PubMed]
- Weber A, Stammberger U, Inci I, et al. Thoracoscopic lobectomy for benign disease--a single centre study on 64 cases. Eur J Cardiothorac Surg 2001;20:443-8. [PubMed]
- Yim AP, Ko KM, Ma CC, et al. Thoracoscopic lobectomy for benign diseases. Chest 1996;109:554-6. [PubMed]
- Sihoe AD, Shiraishi Y, Yew WW. The current role of thoracic surgery in tuberculosis management. Respirology 2009;14:954-68. [PubMed]
- Gonzalez D, Garcia J, Fieira E, et al. Video-assisted thoracoscopic lobectomy in the treatment of intralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg 2011;12:77-9. [PubMed]
- Osaki T, Kodate M, Takagishi T, et al. Unique extralobar sequestration with atypical location and aberrant vessels. Ann Thorac Surg 2010;90:1711-2. [PubMed]
- Kwon YS, Koh WJ, Han J, et al. Clinical characteristics and feasibility of thoracoscopic approach for congenital cystic adenomatoid malformation in adults. Eur J Cardiothorac Surg 2007;31:797-801. [PubMed]
- Yamasaki N, Tagawa T, Nakamura A, et al. Video-assisted thoracoscopic resection for intralobar pulmonary sequestration. Gen Thorac Cardiovasc Surg 2009;57:46-8. [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22. [PubMed]
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [PubMed]
- Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- McKenna RJ Jr, Wolf RK, Brenner M, et al. Is lobectomy by video-assisted thoracic surgery an adequate cancer operation? Ann Thorac Surg 1998;66:1903-8. [PubMed]


