
Keratin 17 is an imaging biomarker in lung cancers
Introduction
Lung cancer is the leading cause of cancer death in men and women worldwide with 5-year survival rate estimated to be 17% (1). However, the National Lung Screening Trial showed a 20% reduction in lung cancer mortality rate highlighting the importance of appropriate, early identification and management of pulmonary nodules (2). Lung cancer detected at an early stage has the highest rate of curative therapy; however, rates of recurrence are still reported at 30% (3). Further, a key aspect of lung cancer screening is lung nodules detection and characterization.
Computed tomographic (CT) features of pulmonary nodules include, size, cavitation, ground glass opacities, fatty components, calcifications and margin features (smooth, lobulated, irregular, spiculated), studies have demonstrated their value in classifying and assessing pulmonary nodules (4,5). Recent studies have shown that CT features, such as ground glass opacities, may have implications for molecular status of the lung cancer (6,7).
The presence of keratin 17 (K17) is associated with high-grade squamous intraepithelial lesions (HSIL) and squamous cell carcinomas of the cervix (8). Cervical squamous cell carcinoma patients with more extensive K17 expression are associated with poor 5-year overall survival rate, with a greater than 11-fold increased mortality rate compared to patients with low/no K17 expression. Studies have also suggested that the K17 may be overexpressed in multiple different cancers including lung cancer (8-10).
The purpose of this study is to determine if K17 is to determine if the overexpression of K17 correlates with imaging features in patients with the lung cancer.
Methods
From 2004–2012 patients were recruited to submit the post-surgical tissue specimens to a tissue bank. We retrospectively collected consecutive patients who consented to have their lung specimens banked who were diagnosed with primary lung cancer. This yielded a total of 204 patients. We then interrogated the picture archive communication system (PACS) for the presence of pre-operative CT images. The remaining cohort was 67 patients. This study was approved by the Institutional Review Board.
Each specimen was then histologically evaluated, graded and evaluated by immunoperoxidase methods for presence or absence K17, as previously described (8). The percentage of tumor cells that expressed K17 was scored by a pathologist with over 25 years of experience. K17 overexpression was then categorized as either, K17 negative, (defined as less than 15% of tumor cells overexpressing K17), or K17 positive (greater than or equal 15% of the tumor cells showing strong staining for K17).
CT feature characterization
Each CT study was evaluated for the outer margin features. More than one feature was able to be assigned to each lung cancer. A primary CT feature and a secondary CT feature were assigned. The primary border characteristic was defined as the CT feature that best describes the lesion which was present in greater than 50% of the tumors border. Four separate CT features were selected to evaluate based on prior studies; these included lobulated, spiculated (irregular), smooth and complex (11-17). Additionally, the location of the tumor within the lung was determined as either peripheral, middle lung or central. The presence or absence of cavitation, calcification, presence of a bronchus in the lesion and pleural tagging were recorded. The extent of ground glass opacity was also determined by measuring the largest single axis of ground glass capacity in millimeters.
For the purposes of this study, peripheral was defined as the primary portion of the tumor within 2 cm of the pleural surface, central was defined as the tumor being within 2 cm of the mediastinal structures/hilar structures and the middle was considered all other areas within the lungs.
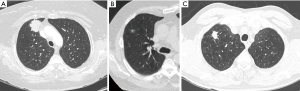
The two interpreting radiologists one with 12-year experience and one with 1-year were blinded to the presence or absence of K17 within this specimen and the specific tissue subtype. Prior to characterization of the lung nodules, the radiologists were trained using five cases that were determined by consensus to represent each imaging characteristics; several examples are seen in Figure 1. Each radiologist interpreted the images independently without knowledge of the K17 status of the patient and without knowledge of the other attendings interpretation. Variance was adjudicated by consensus. All interpretations were performed using high-resolution, three-megapixel color monitors Barco Inc, (Rancho Cordova, CA), all images were displayed on a FDA approved PACs system and viewer, GE PACS IW (GE medical solutions Milwaukee Wisconsin).
Statistics
Inter-observer variation was determined by a kappa statistic. Statistical association of lung nodule characteristics and overexpression of KRT-17 was performed using a Chi-Square test. All calculations were performed using MedCal, (Ostend. Belgium). Parametric data were assessed using a pair two-tailed student t-test. Statistical significance was defined as a P value less than 0.05.
Results
Demographics of patients
The mean patient age was 63.1 years [33–83], 52.2%, (35 of 67) of patients were female. Patient with K17-negative tumors had a mean age of 62.9 years (±10.4), while the mean age of patient with K17-positive tumors was 68.1 years (±8.2) (P=0.87).
The mean tumor size was 2.5 cm (±2.6) with a range from 0.8 to 14.8 cm. Of the 67 lung cancers that were evaluated for the presence of K17, 41 tumors were found not to overexpress K17. Twenty-six patients were found to have evidence of overexpression of K17. Comparing K17 positive and negative tumors, there was no statistical difference between the T-stage (P=0.16) and the N-stage (P=0.14). 59.7% (40/67) had unknown metastatic status (stage Mx) and given the limited imaging set, all potential metastatic disease could not be excluded.
Inter-rater agreement
The readers showed moderate agreement defining primary border characteristic of tumors with a kappa statistic of 0.527 (95% CI, 0.324–0.729). The consensus reads did not favor to either reader. Results of the consensus reads are detailed below and were used for statistical analysis.
Imaging characteristics
K17 negative tumors
K17 negative tumors included 5 squamous cell carcinoma and 36 adenocarcinomas. The mean tumor size of this cohort of patients was 2.17 cm (±0.82). After image analysis, 51.2% of these tumors (21/41) were categorized as spiculated. Peripheral location was common in these tumors with 90.2% (37/41) being defined as peripheral. The extent of ground glass opacity was measured as a mean 2.3 mm (±0.9).
K17 positive tumors
The K17-positive tumors included 26 total tumors (19 squamous cell carcinoma and 7 adenocarcinomas). Peripheral location of these tumors was observed in a total of 18 of these 26 (69.2%) tumors. Three of these 26 tumors were considered central (11.5%), the remaining 5 tumors were defined as mid-lung (19.2%). These features were found not to be statically significantly different from K17 negative tumors (P=0.16). The extent of ground glass opacity was measured at 6.8 mm (±5) this was also not statistically significantly different (P=0.39, see Table 1).

Full table
The border characteristic of lobulation was assigned to 84.6% (22/26) of K17 positive tumors. The border feature of lobulation was associated with K17 positive tumors with a Chi-squared of 7.78 (P=0.02). One tumor was categorized as having calcification and this tumor was positive for K17.
Imaging feature of adenocarcinomas with overexpression of K17 had similar characteristics to the squamous cell carcinomas in this study. Specifically, six out of the seven adenocarcinomas found to over-express K17 were defined as having a lobulated border (85.7%) compared to the squamous cell carcinoma found to overexpress K17 where 16 of the 19 (84.2%) tumors were categorized as having a lobulated border.
Discussion
The CT feature of lobulation is associated with the overexpression of K17. This suggests the potential role of CT as an imaging biomarker which reflects the overexpression of K17 in lung carcinoma. Although statistical significance was not reached, there was a trend for the K17-positive tumors to be in a more central location. Ground glass opacities were not predictive of tumor type. Perhaps a combination of multiple CT features would further be predictive of outcome in these patients. Although, multi-variant analysis was performed the number of studies available was considered too small to reach significance.
Based on the survival data from prior reports K17 positive tumors are clinically more aggressive, further tumors overexpressing K17 may show focal rather than uniform distribution of K17 positive cells (8,10,18). Lobulation has been suggested to be a sign of differential growth pattern of tumors (11-17). The phenotypic expression of these tumors with focal expression of K17 may be lobulation of the tumor margins. Thus, if there is more rapid growth in localized areas of these tumors, perhaps this is associated with the focal areas of K17-overexpression. Additional studies clearly defining the histologic location and extent of K17 positive cells with imaging correlation will be needed to explore this possibility.
Multiple limitations are present in the study, overall the cohort site is relatively small however, multiple areas of statistical significance were reached. Although all imaging studies were of diagnostic quality, there was variability in imaging protocols. Newer CT technique such as dual energy may have provided additional information that was not available in this study. Since this is a reader study and all features were assessed in a semi-quantitative manner, this introduces potential bias. However, the inter-observer agreement in this study was moderate; suggesting that this bias was overcome.
Future studies using high-resolution CT images, dual energy and automated quantitative diagnostic tools to further assess additional textual features are necessary to potentially uncover the imaging biomarkers present within these tumors.
Acknowledgments
The authors would like to acknowledge the Research Histology Core Laboratory, Department of Pathology, Stony Brook Medicine for the technical support of this study.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ammar Chaudhry) for the series “Role of Precision Imaging in Thoracic Disease” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2019.08.33). The series “Role of Precision Imaging in Thoracic Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014.
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee. Advisory Boards, and Participating Institutions.; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Cardinale L, Ardissone F, Novello S, et al. The Pulmonary Nodule: Clinical and Radiological Characteristics Affecting a Diagnosis of Malignancy. Radiol Med 2009;114:871-89. [Crossref] [PubMed]
- Liu Y, Balagurunathan Y, Atwater T, et al. Radiological Image Traits Predictive of Cancer Status in Pulmonary Nodules. Clin Cancer Res 2017;23:1442-49. [Crossref] [PubMed]
- Park J, Kobayashi Y, Urayama KY, et al. Imaging Characteristics of Driver Mutations in EGFR, KRAS, and ALK among Treatment-Naïve Patients with Advanced Lung Adenocarcinoma. PLoS One 2016;11:e0161081. [Crossref] [PubMed]
- Xu Y, Sun H, Zhang Z, et al. Assessment of Relationship Between CT Features and Serum Tumor Marker Index in Early-stage Lung Adenocarcinoma. Acad Radiol 2016;23:1342-48. [Crossref] [PubMed]
- Escobar-Hoyos LF, Yang J, Zhu J, et al. KRT17 in premalignant and malignant squamous lesions of the cervix: proteomic discovery and immunohistochemical validation as a diagnostic and prognostic biomarker. Mod Pathol 2014;27:621-30. [Crossref] [PubMed]
- Chen Y, Cui T, Yang L, et al. The Diagnostic Value of Cytokeratin 5/6, 14, 17, and 18 Expression in Human Non-Small Cell Lung Cancer. Oncology 2011;80:333-40. [Crossref] [PubMed]
- Escobar-Hoyos LF, Shah R, Roa-Pena L, et al. Keratin-17 promotes p27KIP1 nuclear export and degradation and offers potential prognostic utility. Cancer Res 2015;75:3650-62. [Crossref] [PubMed]
- Erasmus JJ, Connolly JE, McAdams P, et al. Solitary Pulmonary Nodules: Part I. Morphologic Evaluation for Differentiation of Benign and Malignant Lesions. Radiographics 2000;20:43-58. [Crossref] [PubMed]
- Gurney JW. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part I. Theory. Radiology 1993;186:405-13. [Crossref] [PubMed]
- Kane GC, Riley CA. Approach to the Pulmonary Nodule. Hospital Physician Board Review Manual 2007;12:1-12.
- Patel VK, Naik SK, Naidich DP, et al. A Practical Algorithmic Approach to the Diagnosis and Management of Solitary Pulmonary Nodules Part 1: Radiologic Characteristics and Imaging Modalities. Chest 2013;143:825-39. [Crossref] [PubMed]
- Sim YT, Poon FW. Imaging of Solitary Pulmonary Nodule—a Clinical Review. Quant Imaging Med Surg 2013;3:316-26. [PubMed]
- Truong MT, Ko JK, Rossi SE, et al. Update in the Evaluation of the Solitary Pulmonary Nodule. Radiographics 2014;34:1658-79. [Crossref] [PubMed]
- Gevaert O, Xu J, Hoang C, et al. Non-Small Cell Lung Cancer: Identifying Prognostic Imaging Biomarkers by Leveraging Public Gene Expression Microarray Data-Methods and Preliminary Results. Radiology 2012;264:387-96. [Crossref] [PubMed]
- Kanda M, Natsuga K, Nishie W, et al. Morphological and genetic analysis of steatocystoma multiplex in an Asian family with pachyonychia congenita type 2 harbouring a KRT17 missense mutation. Br J Dermatol 2009;160:465-68. [Crossref] [PubMed]


