Surgical intervention with lobectomy and mediastinal lymph node dissection is considered the treatment of choice in early stage non-small cell lung cancer (NSCLC) (
1). However, approximately 20-25% of patients with early stage NSCLC are poor surgical candidates for lobectomy because of concomitant severe cardiac or pulmonary co-morbidities. For these patients, conventional radiotherapy with 60 to 70 Gy delivered in 30-35 fractions over a 6-7-week period generally resulted in poor 20-40% 3-year and 10-30% 5-year survival rates (
2,
3). This inadequate tumor control is mainly due to the insufficient tumor dose that is limited by normal tissue toxicity and possible target-miss caused by tumor mobility.
Stereotactic body radiation therapy (SBRT), or stereotactic ablative radiotherapy (SABR), has been emerging as an excellent alternative for medically inoperable early stage NSCLC patients. Conceptually derived from cranial stereotactic radiosurgery, the planning and delivery of SBRT is characterized by highly target-conformal dose distributions with steep dose gradients towards normal tissues, allowing the administration of potent tumor-ablative radiation doses. In lung SBRT, a total of 45-50 Gy of radiation is delivered in 3-5 fractions over a 10-20 days’ duration. Calculated by LQ with α/β =10 Gy, a less than 5 cm tumor is generally treated with higher than 100 biologically equivalent dose (BED). Available data demonstrated an impressive 80-95% local tumor control at 2-5 years and good lung function preservation (
3-
6). The recently published RTOG 0236 phase II study demonstrated 3-years 98% local tumor control and 56% survival (
7). This result is quite comparable to the reported 53% 5-year survival with surgical resection, based on thousands of patients in the International Association for the Study of Lung Cancer Staging Project (
8). Lung SBRT is comparably superior than radiofrequency ablation (RFA), an alternative invasive procedure with a moderate 60% tumor control rate for less than 3 cm tumors, but is also associated with much higher procedure-related morbidities, mainly caused by pneumothorax and hemorrhage (
9).
How to accurately locate small pulmonary targets for lung SBRT is the subject of one article published in this issue of Journal of Thoracic Disease. In their study, Shen
et al. investigated the application of double CT imaging to measure the respiratory movement of small pulmonary tumors during SBRT (
10). A total of 122 small pulmonary tumors in 45 patients were measured. Four-slice spiral CT scans were conducted twice in all patientsonce
each at the end of quiet inhalation and of exhalation, and
three times in 17 patients - with one additional free breathing
image. The displacement of the tumor center in three directions
was measured. The study showed an overall 3D motion of
10.10±7.16 mm in 122 tumors, with 1.96±2.03, 5.19±4.69
and 7.38±6.48 mm in the X, Y and Z directions, respectively.
The extent of tumor motion was influenced by the pulmonary
location of individual tumor: greater motion was noted in
tumors in the lower, left and anterior locations than in the upper,
right and posterior locations. In contrast to 4-dimentional CT,
this is a relatively less expansive, yet practical method for target
localization. Their conclusions are in general agreement with
published results (
11).
The success of lung SBRT relies largely on accurate target
localization, which enables precise ablative radiotherapy to
target while maximizing the spared surrounding normal tissues
from treatment-related side effects. It is an eminent observation
that small-sized lung tumors are moving targets, which changes
not only their locations, but also their shapes and volumes as
the lung inflates and deflates. In addition, respiratory-induced
motion can cause severe geometrical distortion of tumors
and normal tissues in free breathing CT scanning. A variety of
methods and techniques have been used to determine the exact
location of a moving target inside of lung. Voluntary breathholding
during imaging and treatment represents one simple,
but often problematic approach in many lung cancer patients
due to poor lung function and anxiety issues. Four-dimensional
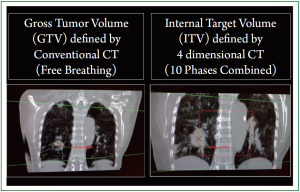
CT (4D-CT) is currently considered a standard methodology
to reduce motion artifact and allow accurate determination
of internal target volume (ITV; Figure 1). In 4D-CT, an oversampled
spiral CT scan with continuous slices is acquired
simultaneously, while the respiratory motion (arbitrarily divided
into 10 phases) is recorded by an infrared camera-based motiontracking
system (
12). 4D-CT is capable of accurately defining
the location and volume of the tumor and its surrounding organs
over time during breathing cycles.
A famous Chinese idiom - “A Millimeter Miss is as Good as a
Thousand Miles” applies to how in medicine, even slightly subtle
errors may lead to huge consequences. Target localization for
lung SBRT represents one such situation, in which inaccuracy in
millimeters may result in the dire consequence of treatment failure.