|
Evaluation
Evaluation of technical success and rejection
After technically successful lung transplantation the graft
should be well aerated and perfused, even in the face of mild
acute rejection. As mentioned above, unlike cardiac allografts,
where early technical failure can be manifested by cessation
of heart beat, the evaluation of the technical success of lung
transplantation requires either gross inspection after sacrifice or
radiographic imaging. Conventional imaging modalities, such
as X-rays, lung perfusion scintigraphy or angiography may be useful in humans and large animals, but are not practical for large
throughput experiments in mice ( 44- 48). Either the resolution
of these techniques is not suitable to evaluate subtle pathological
changes, or the techniques cannot be performed on a serial
basis in the same animal ( 36). Greschus suggested Flat-Panel
Volumetric Computer Tomography (fpVCT) as a precise tool
to assess the success of rat orthotopic lung transplantation that
can be used to follow the process of graft rejection with very high
spatial resolution ( 36). Such a technique, however, might not be
applicable to the mouse due to its small size. We have recently
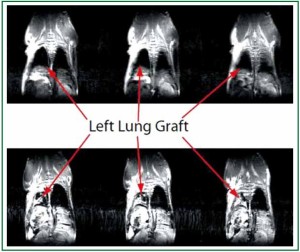
focused on small animal MRI to evaluate pulmonary pathology
and have found this technique to be a highly reproducible
non-invasive approach to visualize anatomic pathology of the
lung such as pulmonary collapse due to either advanced acute
rejection or atelectasis ( 29) (Figure 4). However, we realize that
this modality might not be easily accessible to all laboratories
and thus to date there still is no easily accessible, effective
and precise imaging modality to evaluate the grafted lung for
technical success or rejection. Our current practice thus relies on
grading rejection based on strict histologic criteria, which mirror
human graft evaluation. In 1990, the International Society for
Heart and Lung Transplantation (ISHLT) adopted a “Working
Formulation of the Standardization of the Nomenclature in the
Diagnosis of Lung Rejection,” which was revised in 1996 and
again in 2007 ( 49). We have thus adapted this grading scale for
the mouse model (Table 1). The development of better imaging
modalities in the future may facilitate serial monitoring of graft
outcome and early detection of technical failures.
| Table 1. Classification and grading of pulmonary allograft rejection. |
| A: Acute rejection |
with/without |
B: Airway inflammation-lymphocytic bronchitis/bronchiolitis |
| Grade 0: None |
|
Grade X: Ungradeable |
| Grade 1: Minimal |
|
Grade 0: None |
| Grade 2: Mild |
|
Grade 1R: Low grade |
| Grade 3: Moderate |
|
Grade 2R: High grade |
| Grade 4: Severe |
|
|
| C: Chronic airway rejection: bronchiolitis obliterans |
| 0: Absent |
| 1: Present |
| D: Chronic vascular rejection: accelerated graft vascular sclerosis |
| “R” denotes revised grade to avoid confusion with 1996 scheme. Stewart, S, Fishbein, MC, Snell, GI, et al. Revision of the 1996 working
formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007; 26:1229 |
Evaluation of graft function
Mouse orthotopic lung transplantation is a very useful model
not only because it mimics human lung transplantation, but
because the investigator can collect samples that mimic clinical
investigations of human lung transplant recipients, such as
arterial blood gases (ABGs), bronchioalveolar lavages (BALs),
and lung parenchymal tissue. The most important tests, such as
spirometry and plethysmography have been reported by some
groups ( 21, 50), but are not currently routinely used by us. To
date our laboratory has relied mostly on arterial blood gas (ABG)
measurement of a mouse supported solely by the lung graft after
hilar clamping of the native lung to evaluate function as well
as bronchoalveolar lavage, wet dry ratio, histology, FACS, and
immunohistochemistry. We have focused on these tests as they
mirror the techniques of periopertaive graft assessment used in
the clinics. Some of these techniques are described below. Arterial Blood Gas measurement(ABG)
Our laboratory has relied on ABG measurement to assess graft
function ( 1, 6, 16, 18, 19). (I) Anesthetize the recipient mouse with an intraperitoneal
(i.p) injection of Ketamine (5-6 μg/g) and Xylazine (7-8 μg/g).
This is about 2/3 of the regular dose used during the actual
transplantation procedure as often after transplantation the
recipient cannot tolerate a regular dose of anesthetics ( 5). (II) Initiate mechanical ventilation of both lungs with 100%
FiO2 for 4 minutes prior to hilar clamping.
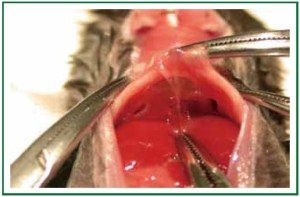
(III) Occlude the hilum of the native non-transplanted lung
(use either a clip or a 6-0 silk tie. Figures 6, 7) and ventilate 4 to
10 more minutes prior to drawing blood.
Here, the microsurgeon needs to detach the right lower
lobe from the esophagus very carefully and free the entire right
lung (in case of left lung transplantation) from connective
tissue without bleeding. The total circulating blood volume in
the mouse is only around 2 mL (6-8% of whole body weight),
and literally any blood loss will affect hemodynamics, result
in circulatory instability and impact the ABG measurement.
Ventilate both lungs for 4 minutes before occluding the native
right lung in order to assess the function of the graft. The
duration of single lung ventilation prior to drawing ABG is controversial and must be tailored based on experimental
conditions ( 1, 26). After transplantation either ischemiareperfusion
injury or graft rejection can cause severe lung graft
dysfunction. Poor oxygenation as a result of this type of injury
can lead to myocardial ischemia, heart failure, and death in
approximately 10 minutes. As mice consume large quantities
of O 2 even at rest ( 31, 33), the fall in PaO2 and the associated
oxyghemoglobin desaturation occurs fairly rapidly ( 51). Thus,
in our experience drawing the ABG after 4 to 10 minutes of
single lung ventilation will allow for a sensitive assessment of
graft function with reliable and reproducible data obtained
by matching the period of single lung ventilation between
experimental and control groups. (IV) Use a 1mL heparin coated syringe with 25 G needle to
draw blood from the left ventricle or ascending aorta to measure
ABG.
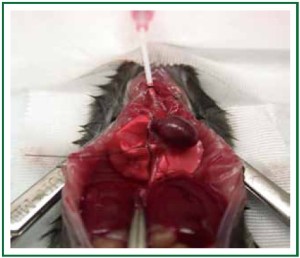
Broncho-alveolar lavage (BAL)
BAL is used to analyze the influx of inflammatory cells into the
airways after human lung transplantation ( 52) and has been
used by our laboratory to assess lung grafts ( 3, 53). Using current
techniques BAL fluid collection originates from both right and
left lung as the lavage is performed with an open chest while
intubating the main trachea (Figure 8). The advantage of this
technique is that it is easy to perform but the disadvantage is that
it mixes the airway cell infiltrates of the native and transplanted
lung. Nevertheless, our data describe that airway inflammatory
cells, such as neutrophils, correlate with tissue infiltration in
the transplanted lung ( 4) and thus we have routinely utilized
tracheal BAL to sample the airways and are currently developing
methods to use a longer catheter that can be inserted into either the left or right lung selectively. Alternatively one can occlude
the native lung in order to collect the BAL sample exclusively
from the graft. Two-photon microscopy
As the lung is constantly exposed to both innocuous and potentially
noxious antigens, a thorough understanding of both innate and
adaptive immune responses in this organ is essential ( 30). Twophoton
microscopy has evolved into a powerful tool that
can allow for observation of cellular interactions in real time.
Such a technique has allowed us to substantially extend our
understanding of immune responses ( 54- 59). Recently, our
group has expanded 2-photon microscopy to the study of
the inflammatory responses in the lung, which has allowed
us to study ischemia reperfusion injury in vivo ( 28, 55, 56).
By relying on this approach we were able to demonstrate
that, contrary to popular belief, monocytes coordinate the
transendothelial migration of neutrophils into inflamed tissue.
We determined that depletion of blood monocytes impairs
neutrophil recruitment to the lung, which could have important
implications for the design of therapeutic strategies to treat
inflammatory lung diseases ( 28). Based on this experience, we
have expanded the use of intravital two-photon microscopy to
investigate cellular trafficking behavior after lung transplantation.
|
|
Scientific uses of mouse orthotopic lung transplantation
Lung transplantation is an established therapy for a variety of
end-stage pulmonary disease. Importantly, long-term outcome
after lung transplantation are far worse than those of other solid
organs ( 60). Immunologic and non-immunologic mechanisms
that contribute to acute and chronic graft lung dysfunction
remain poorly understood and the mouse lung transplantation
model presents a unique tool that can allow us to study innate
and adaptive immune responses after lung transplantation. Ischemia-reperfusion (I-R) injury-mediated primary graft
dysfunction (PGD)
PGD is a form of acute lung injury that results from
inflammatory changes induced by I-R injury ( 61). PGD is graded
based on PaO2/FiO2 (P/F) ratio and radiographic infiltrates
assessed at several time points up to 72 hours after transplantation
(Table 2). By definition this form of injury arises within the first
72 hours following lung transplantation and is a leading cause
of early morbidity and mortality after transplantation. PGD is
characterized by impaired oxygenation and pulmonary edema
and affects up to 80% of all lung transplant recipients (62-68).
In addition, PGD has been linked to the development of chronic
allograft rejection manifested by bronchiolitis obliterans (69).
Thus, a better knowledge of the pathophysiology of I-R injury
should facilitate a better understanding of PGD. Biomarker
phenotyping should become possible in order to develop
novel therapeutics and reduce the impact of PGD on lung
transplant outcomes ( 62). Neutrophils and factors that control
their production and activation play a critical role in I-R injury.
Over the last 5 years using the mouse model of orthotopic lung transplantation our group has focused heavily on this cell type.
We have delineated that neutrophils isolated from the airways
of lung transplantation recipients stimulate donor dendritic
cells (DCs) in a contact-dependent fashion to augment their
production of IL-12 and expand alloantigen-specific IFN-γ(+) T
cells. DC IL-12 expression is largely regulated by degranulation
and induced by TNF-α associated with the neutrophil plasma
membrane. Extended cold ischemic graft storage enhances
G-CSF-mediated granulopoiesis and neutrophilic graft
infiltration, resulting in exacerbation of I-R injury after lung
transplantation. I-R injury prevents immunosuppressionmediated
acceptance of mouse lung allografts unless G-CSFmediated
granulopoiesis is inhibited ( 19). In addition, we also
identified that transcriptional coregulator B cell leukemia/
lymphoma 3 (Bcl3) limits granulopoiesis under inflammatory
conditions. Bcl3-deficient myeloid progenitors demonstrated
an enhanced capacity to proliferate and differentiate into
granulocytes following G-CSF stimulation, whereas the
accumulation of Bcl3 protein attenuated granulopoiesis in an
NF-κB p50-dependent manner ( 70). Future experiments will
focus on therapeutic strategies to modulate the activation of and
degranulation of neutrophils in order to ameliorate pulmonary
graft injury.
| Table 2. ISHLT PGD grading schema. |
| Grade |
PaO2/FiO2 |
Radiographic Infiltrates Consistent with Pulmonary Edema |
| 0 |
>300 |
Absent |
| 1 |
>300 |
Present |
| 2 |
200-300 |
GPresent |
| 3 |
<200 |
Present |
| Time points for assessment: T (0 to within 6 hours of reperfusion, 24, 48, and 72 hours). Data from Christie JD, Carby M, Bag R, et al.
Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International
Society for Heart and Lung Transplantation. J Heart Lung Transplant 2005;24:1458. |
IAcute cellular rejection (ACR)
According to the ISHLT Registry, 36% of lung transplant
recipients experience at least one episode of ACR within
the first year after transplantation ( 71). Risk factors for ACR
remain poorly defined. The degree of major histocompatibility
antigen (MHC) discordance between donor and recipient has
been identified as a risk factor in some studies ( 67- 69, 72). The
mouse model of lung transplantation offers an ideal platform
to study ACR as inbred mouse strains have well defined MHC
antigens allowing for evaluation of ACR in fully mismatched,
minor antigen mismatched, and partially matched (by using F1
crosses as graft donors for example) situations. Since the mouse
lung transplantation model was established, we have verified
histopathologically and flow cytometrically that this new animal
model can recapitulate acute lung allograft rejection successfully
( 1). We have also demonstrated that, similar to other organs
( 73), treatment of lung allografts with anti-CD28-B7 and CD40-
CD40 Ligand co-stimulatory blockade can prolong allograft
survival indefinitely in several strain combinations ( 7). Dodd-o
and colleagues found that anti-CD154 antibody therapy alone
is sufficient to attenuate ACR in an MHC mismatched mouse
orthotropic lung transplant model. Improved lung allograft
acceptance in anti-CD154 Ab treated recipients was associated
with abrogated CD8+ and CD4+ allospecific effector responses
and increased frequencies of CD4+CD25+Foxp3+ regulatory
T-cells in the lung allografts ( 74). Our group has also provided
evidence that pulmonary nonhematopoietic cells, through
their expression of MHC-II, play a critical role in downregulating
CD4+ T cell-mediated immune responses in vivo ( 2, 4). Obviously,
this new mouse model will allow for the design of novel studies
that elucidate mechanisms of ACR and provide rationale for the
development of therapeutic approaches. Chronic rejection – Obliterative bronchiolitis (OB)
OB is a form of chronic rejection specific to the lung and is
characterized by progressive fibrosis and obliteration of the small
and medium-sized airways of the donor lung ( 75). The mechanisms
leading to the development of this condition still remain unclear
( 76). Although lymphocytes are observed in the bronchial wall, the
prominent cell type found in the BAL is neutrophils ( 77). Recently,
evidence suggests the involvement of multiple factors such excessive
activation of innate immune responses, abnormal angiogenesis and
failure of appropriate epithelial regeneration and fibroproliferative
tissue remodeling ( 78). Laboratory experimentation using animal
models forms an important component of a “bench-to-bedsideto-
bench” approach that can both increase our understanding
and lead to the development of novel therapeutic strategies for
this ( 76). Of the several different animal OB models available, each
has advantages and limitations. There is not an “ideal” model that precisely reproduces what happens to humans after lung
transplantation. The lesions of OB are thought to represent the
shared histological outcome of injury to the airway epithelium
and subcellular matrix by an array of immune and inflammatory
insults. From a clinical standpoint, OB remains heterogeneous,
varying both in timing of onset after transplantation and
aggressiveness in clinical course ( 79). So, it is not practical or
scientifically desirable to test multiple contributing factors at
the same time. Pulmonary chronic rejection shares features
of chronic rejection observed in other solid organ. Chronic
rejection in all organs mainly manifests as fibrosis in the graft
resulting in loss of function and eventually grafts loss ( 80).
There are several immunologic antigens involved in this fibrosis,
including major and minor histocompatibility antigens ( 81)
as well as self-antigens ( 82) as both can emerge as targets of
immune responses after transplantation ( 83- 85). In theory the orthotopic mouse lung transplantation model
has great advantages for studying the pathogenesis of OB as
it offers a physiological model of the human lung transplant
environment. Lung function and BAL can be assessed at
various times, various drugs can be tested for efficacy, and
transgenic and knock-out strains are available to model human
diseases. However, several physiologic factors in the mouse
lung anatomy and physiology create potential obstacles in the
study of OB. The initial OB process starts with a lymphocytic
infiltrate of the sub-mucosa of the airways followed by
the migration of the lymphocytes through the basement
membrane into the epithelium ( 86). At this site, epithelial cell
necrosis occurs with denudation of mucosa. In the mouse lung,
there is lack of submucosal glands in the bronchioles, but a
high numbers of locally resident Clara cells. One of the main
functions of Clara cells is to protect the bronchiolar epithelium
from injury. They accomplish this by secreting a variety of
proteins, including Clara cell secretory protein (CCSP) and a
solution similar to lung surfactant. They are also responsible
for detoxifying inhaled harmful substances. Clara cells also act
as a stem cell and multiply to differentiate into ciliated cells
that can regenerate bronchiolar epithelium. This is a possible
reason why mouse airway epithelium remains intact and fully
differentiated in lung allografts, despite profound vascular
rejection ( 7). Since 2007, a few separate groups have tried to
establish an OB model in the mouse. Although two groups
have found OB lesions by histology, the experimental design
and the histological features still need further clarification
( 9, 27). Currently we are focusing on three factors that may
influence the development of OB including: (I) donor and
recipient strain combinations; (II) time period necessary
to see chronic rejection in the mouse and; (III) the form of
immunosuppression that may allow for OB to develop. Once
established and reproducible, such a model could hold great
promise for further mechanistic studies and may be used to
accelerate the development of new strategies for the prevention
or treatment of pulmonary chronic rejection. Studies of Non-hematopoietic stromal cells
While bone marrow-derived hematopoietic cells, such as
dendritic cells, play a critical role in pulmonary physiology
( 87- 92), we as well as others have focused on the contribution
of non-hematopoietic cells in pulmonary immune responses
( 4, 93). Traditional methods for separating the physiology
of hematopoietic from non-hematopoietic cells involves the
creation of bone marrow chimeras by lethal irradiation of a
recipient mouse followed by reconstitution with bone marrow
from a mutant strain ( 94). Donor irradiation, however, may
change the physiology of non-hematopoietic cells and bone
marrow chimeras can also suffer from autoimmune disease ( 4).
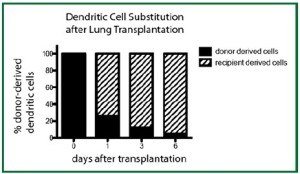
We have recently demonstrated that the transplantation of a
left lung into a congenic host leads to the rapid substitution
of donor-derived hematopoietic cells with those of the host,
leading to the creation of a “chimeric lung graft” in an otherwise
immunocompetent host. Furthermore, such substitution occurs
for multiple types of hematopoietic cells such as T cells ( 4, 5)
and dendritic cells (Figure 9). Such a model can thus be used
to study both immunology and physiology of pulmonary nonhematopoietic
cells in the absence of irradiation. |
|
References
- Okazaki M, Krupnick AS, Kornfeld CG, et al. A mouse model of orthotopic
vascularized aerated lung transplantation. Am J Transplant 2007;7:1672-9.
- Gelman AE, Okazaki M, Sugimoto S, et al. CCR2 regulates monocyte
recruitment as well as CD4 T1 allorecognition after lung transplantation.
Am J Transplant 2010;10:1189-99.
- Kreisel D, Lai J, Richardson SB, et al. Polarized alloantigen presentation by
airway epithelial cells contributes to direct CD8+ T cell activation in the
airway. Am J Respir Cell Mol Biol 2011;44:749-54.
- Kreisel D, Richardson SB, Li W, et al. Cutting edge: MHC class II
expression by pulmonary nonhematopoietic cells plays a critical role in
controlling local inflammatory responses. J Immunol 2010;185:3809-13.
- Krupnick AS, Lin X, Li W, et al. Orthotopic mouse lung transplantation
as experimental methodology to study transplant and tumor biology. Nat
Protoc 2009;4:86-93.
- Li W, Sugimoto S, Lai J, et al. Orthotopic vascularized right lung
transplantation in the mouse. J Thorac Cardiovasc Surg 2010;139:1637-43.
- Okazaki M, Gelman AE, Tietjens JR , et al. Maintenance of airway
epithelium in acutely rejected orthotopic vascularized mouse lung
transplants. Am J Respir Cell Mol Biol 2007;37:625-30.
- Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular
immunity to collagen type V predisposes to obliterative bronchiolitis in
human lung transplants. J Clin Invest 2007;117:3498-506.
- Fan L, Benson HL, Vittal R, et al. Neutralizing IL-17 prevents obliterative
bronchiolitis in murine orthotopic lung transplantation. Am J Transplant
2011;11:911-22.
- Shilling RA, Wilkes DS. Role of Th17 cells and IL-17 in lung transplant
rejection. Semin Immunopathol 2011;33:129-34.
- Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a
focus on immunity to type V collagen in the pathogenesis of lung transplant
rejection. Am J Physiol Lung Cell Mol Physiol 2004;286:L1129-39.
- Wilkes DS. Chronic lung allograft rejection and airway microvasculature: is
HIF-1 the missing link? J Clin Invest 2011;121:2155-7.
- Yasufuku K, Heidler KM, Woods KA, et al. Prevention of bronchiolitis
obliterans in rat lung allografts by type V collagen-induced oral tolerance.
Transplantation 2002;73:500-5.
- Gelman AE, Okazaki M, Lai J, et al. CD4+ T lymphocytes are not necessary
for the acute rejection of vascularized mouse lung transplants. J Immunol
2008;180:4754-62.
- Gelman AE, Li W, Richardson SB, et al. Cutting edge: Acute lung allograft
rejection is independent of secondary lymphoid organs. J Immunol
2009;182:3969-73.
- Huang HJ, Sugimoto S, Lai J, et al. Maintenance of IKKbeta activity
is necessary to protect lung grafts from acute injury. Transplantation
2011;91:624-31.
- Okazaki M, Sugimoto S, Lai J, et al. Costimulatory blockade-mediated lung
allograft acceptance is abrogated by overexpression of Bcl-2 in the recipient.
Transplant Proc 2009;41:385-7.
- Sugimoto S, Lin X, Okazaki M, et al. Monocyte differentiation is controlled
by MyD88 after mouse orthotopic lung transplantation. Transplant Proc
2009;41:388-90.
- Kreisel D, Sugimoto S, Zhu J, et al. Emergency granulopoiesis promotes
neutrophil-dendritic cell encounters that prevent mouse lung allograft
acceptance. Blood 2011;118:6172-82.
- Jungraithmayr W, Weder W. The Technique of Orthotopic Mouse Lung
Transplantation as a Movie-Improved Learning by Visualization. Am J
Transplant 2012;12:1624-6.
- De Vleeschauwer S, Jungraithmayr W, Wauters S, et al. Chronic rejection
pathology after orthotopic lung transplantation in mice: the development
of a murine BOS model and its drawbacks. PLoS ONE 2012;7:e29802.
- Jungraithmayr W, De Meester I, Matheeussen V, et al. CD26/DPP-4
inhibition recruits regenerative stem cells via stromal cell-derived factor-1
and beneficially influences ischaemia-reperfusion injury in mouse lung
transplantation. Eur J Cardiothorac Surg 2012;41:1166-73.
- Jungraithmayr W, Draenert A, Marquardt K, et al. Ultrastructural changes
in acute lung allograft rejection: novel insights from an animal study. J
Heart Lung Transplant 2012;31:94-100.
- Iken K, Liu K, Liu H, et al. IDO and Metabolites Protect Mouse Lung
Allografts and Impair Calcium Mobilization of T Cells. Am J Respir Cell
Mol Biol 2012. [Epub ahead of print].
- Waki N, Yamane M, Yamamoto S, et al. Egr1: a novel target for ameliorating
acute allograft rejection in an experimental lung transplant model. Eur J
Cardiothorac Surg 2012;41:669-75.
- Jungraithmayr WM, Korom S, Hillinger S, et al. A mouse model of
orthotopic, single-lung transplantation. J Thorac Cardiovasc Surg
2009;137:486-91.
- Kreisel D, Gelman AE, Palmer SM. In pursuit of new experimental models
of obliterative bronchiolitis. Am J Transplant 2011;11:882-3.
- Kreisel D, Nava RG, Li W, et al. In vivo two-photon imaging reveals
monocyte-dependent neutrophil extravasation during pulmonary
inflammation. Proc Natl Acad Sci USA 2010;107:18073-8.
- Krupnick AS, Tidwell VK, Engelbach JA, et al. Quantitative monitoring
of mouse lung tumors by magnetic resonance imaging. Nat Protoc
2012;7:128-42.
- Nava RG, Li W, Gelman AE, et al. Two-photon microscopy in pulmonary
research. Semin Immunopathol 2010;32:297-304.
- Kling MA. A review of respiratory system anatomy, physiology, and disease
in the mouse, rat, hamster, and gerbil. Vet Clin North Am Exot Anim Pract
2011;14:287-337.
- Morgan GE, Mikhail MS, Murray MJ. Clinical anesthesiology. 4th ed. New
York: Lange Medical Books/McGraw Hill, Medical Pub. Division; 2006.
xiv, 1105 p.p.
- Irvin CG, Bates JH. Measuring the lung function in the mouse: the
challenge of size. Respir Res 2003;4:4.
- Knudsen L, Weibel ER, Gundersen HJ, et al. Assessment of air space size
characteristics by intercept (chord) measurement: an accurate and efficient
stereological approach. J Appl Physiol 2010;108:412-21.
- Gomes RF, Bates JH. Geometric determinants of airway resistance in two
isomorphic rodent species. Respir Physiol Neurobiol 2002;130:317-25.
- Greschus S, Kuchenbuch T, Plotz C, et al. Monitoring of experimental
rat lung transplants by high-resolution flat-panel volumetric computer
tomography (fpVCT). J Invest Surg 2009;22:69-75.
- Santacruz JF, Mehta AC. Airway complications and management after lung
transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc
2009;6:79-93.
- Tendulkar RD, Fleming PA, Reddy CA, et al. High-dose-rate endobronchial
brachytherapy for recurrent airway obstruction from hyperplastic
granulation tissue. Int J Radiat Oncol Biol Phys 2008;70:701-6.
- Kennedy AS, Sonett JR, Orens JB, et al. High dose rate brachytherapy to
prevent recurrent benign hyperplasia in lung transplant bronchi: theoretical
and clinical considerations. J Heart Lung Transplant 2000;19:155-9.
- Shennib H, Massard G. Airway complications in lung transplantation. Ann
Thorac Surg 1994;57:506-11.
- Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk factors for
airway complications within the first year after lung transplantation. Eur J
Cardiothorac Surg 2007;31:703-10.
- Mulligan MS. Endoscopic management of airway complications after lung
transplantation. Chest Surg Clin N Am 2001;11:907-15.
- Choong CK, Sweet SC, Zoole JB, et al. Bronchial airway anastomotic
complications after pediatric lung transplantation: incidence, cause,
management, and outcome. J Thorac Cardiovasc Surg 2006;131:198-203.
- Marck KW, Piers DA, Wildevuur CR. Lung transplantation in the rat: II.
lung perfusion scintigraphy in normal and left lung-transplanted rats. Ann
Thorac Surg 1982;34:81-8.
- Marck KW, Prop J, Wildevuur CR. Lung transplantation in the rat. III.
Functional studies in iso- and allografts. J Surg Res 1983;35:149-58.
- Zweers N, Petersen AH, van der Hoeven JA, et al. Donor brain
death aggravates chronic rejection after lung transplantation in rats.
Transplantation 2004;78:1251-8.
- Reis A, Giaid A, Serrick C, et al. Improved outcome of rat lung
transplantation with modification of the nonsuture external cuff technique.
J Heart Lung Transplant 1995;14:274-9.
- Yuh DD, Gandy KL, Morris RE, et al. Leflunomide prolongs pulmonary
allograft and xenograft survival. J Heart Lung Transplant 1995;14:1136-44.
- Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working
formulation for the standardization of nomenclature in the diagnosis of
lung rejection. J Heart Lung Transplant 2007;26:1229-42.
- Vanoirbeek JA, Rinaldi M, De Vooght V, et al. Noninvasive and invasive
pulmonary function in mouse models of obstructive and restrictive
respiratory diseases. Am J Respir Cell Mol Biol 2010;42:96-104.
- Lee EJ, Woodske ME, Zou B, et al. Dynamic arterial blood gas analysis in
conscious, unrestrained C57BL/6J mice during exposure to intermittent
hypoxia. J Appl Physiol 2009;107:290-4.
- Heron M, Grutters JC, Ten Dam-Molenkamp KM, et al. Bronchoalveolar
lavage cell pattern from healthy human lung. Clin Exp Immunol
2012;167:523-31.
- Vikis HG, Gelman AE, Franklin A, et al. Neutrophils are required for
3-methylcholanthrene-initiated, butylated hydroxytoluene-promoted lung
carcinogenesis. Mol Carcinog 2011. [Epub ahead of print].
- Miller MJ, Wei SH, Parker I, et al. Two-photon imaging of lymphocyte
motility and antigen response in intact lymph node. Science
2002;296:1869-73.
- Cahalan MD, Parker I, Wei SH, et al. Two-photon tissue imaging: seeing
the immune system in a fresh light. Nat Rev Immunol 2002;2:872-80.
- Cahalan MD, Parker I, Wei SH, et al. Real-time imaging of lymphocytes in
vivo. Curr Opin Immunol 2003;15:372-7.
- Miller MJ, Wei SH, Cahalan MD, et al. Autonomous T cell trafficking
examined in vivo with intravital two-photon microscopy. Proc Natl Acad
Sci USA 2003;100:2604-9.
- Miller MJ, Hejazi AS, Wei SH, et al. T cell repertoire scanning is promoted
by dynamic dendritic cell behavior and random T cell motility in the lymph
node. Proc Natl Acad Sci USA 2004;101:998-1003.
- Miller MJ, Safrina O, Parker I, et al. Imaging the single cell dynamics of
CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med
2004;200:847-56.
- Trulock EP, Edwards LB, Taylor DO, et al. Registry of the International
Society for Heart and Lung Transplantation: twenty-third official adult
lung and heart-lung transplantation report--2006. J Heart Lung Transplant
2006;25:880-92.
- Lee JC, Christie JD. Primary graft dysfunction. Clin Chest Med
2011;32:279-93.
- Christie JD, Bavaria JE, Palevsky HI, et al. Primary graft failure following
lung transplantation. Chest 1998;114:51-60.
- Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary
graft failure following lung transplantation. Chest 2003;124:1232-41.
- Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med
1999;340:1081-91.
- Christie JD, Van Raemdonck D, de Perrot M, et al. Report of the ISHLT
Working Group on Primary Lung Graft Dysfunction part I: introduction
and methods. J Heart Lung Transplant 2005;24:1451-3.
- Arcasoy SM, Fisher A, Hachem RR, et al. Report of the ISHLT Working
Group on Primary Lung Graft Dysfunction part V: predictors and
outcomes. J Heart Lung Transplant 2005;24:1483-8.
- Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on
Primary Lung Graft Dysfunction part II: definition. A consensus statement
of the International Society for Heart and Lung Transplantation. J Heart
Lung Transplant 2005;24:1454-9.
- King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly
impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg
2000;69:1681-5.
- Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung
allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir
Crit Care Med 2007;175:507-13.
- Kreisel D, Sugimoto S, Tietjens J, et al. Bcl3 prevents acute inflammatory
lung injury in mice by restraining emergency granulopoiesis. J Clin Invest
2011;121:265-76.
- Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med
2011;184:159-71.
- Palmer SM, Burch LH, Davis RD, et al. The role of innate immunity in
acute allograft rejection after lung transplantation. Am J Respir Crit Care
Med 2003;168:628-32.
- Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin
and cardiac allografts after blocking CD40 and CD28 pathways. Nature
1996;381:434-8.
- Dodd-o JM, Lendermon EA, Miller HL, et al. CD154 blockade abrogates
allospecific responses and enhances CD4(+) regulatory T-cells in mouse
orthotopic lung transplant. Am J Transplant 2011;11:1815-24.
- Sato M, Keshavjee S, Liu M. Translational research: animal models of
obliterative bronchiolitis after lung transplantation. Am J Transplant
2009;9:1981-7.
- De Vleeschauwer S, Vanaudenaerde B, Vos R, et al. The need for a new
animal model for chronic rejection after lung transplantation. Transplant
Proc 2011;43:3476-85.
- Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome
2001: an update of the diagnostic criteria. J Heart Lung Transplant
2002;21:297-310.
- Sato M, Keshavjee S. Bronchiolitis obliterans syndrome: alloimmunedependent
and -independent injury with aberrant tissue remodeling. Semin
Thorac Cardiovasc Surg 2008;20:173-82.
- Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier
for lung transplantation. Chest 2011;140:502-8.
- Rocha PN, Plumb TJ, Crowley SD, et al. Effector mechanisms in transplant
rejection. Immunol Rev 2003;196:51-64.
- Dierselhuis M, Goulmy E. The relevance of minor histocompatibility
antigens in solid organ transplantation. Curr Opin Organ Transplant
2009;14:419-25.
- Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I
induce autoimmunity: role in the pathogenesis of chronic rejection. J
Immunol 2009;182:309-18.
- Rolls HK, Kishimoto K, Dong VM, et al. T-cell response to cardiac myosin
persists in the absence of an alloimmune response in recipients with
chronic cardiac allograft rejection. Transplantation 2002;74:1053-7.
- Barber LD, Whitelegg A, Madrigal JA, et al. Detection of vimentin-specific
autoreactive CD8+ T cells in cardiac transplant patients. Transplantation
2004;77:1604-9.
- Carter V, Shenton BK, Jaques B, et al. Vimentin antibodies: a non-HLA
antibody as a potential risk factor in renal transplantation. Transplant Proc
2005;37:654-7.
- Yousem SA. Lymphocytic bronchitis/bronchiolitis in lung allograft
recipients. Am J Surg Pathol 1993;17:491-6.
- Grayson MH, Holtzman MJ. Emerging role of dendritic cells in respiratory
viral infection. J Mol Med (Berl) 2007;85:1057-68.
- Hammad H, Lambrecht BN. Lung dendritic cell migration. Adv Immunol
2007;93:265-78.
- Ray P, Krishnamoorthy N, Ray A. Emerging functions of c-kit and its ligand
stem cell factor in dendritic cells: regulators of T cell differentiation. Cell
Cycle 2008;7:2826-32.
- Suzuki T, Chow CW, Downey GP. Role of innate immune cells and their
products in lung immunopathology. Int J Biochem Cell Biol 2008;40:1348-61.
- Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as
master regulators of allergic airway inflammation. Lancet 2010;376:835-43.
- Chiappori AA, Soliman H, Janssen WE, et al. INGN-225: a dendritic
cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: observed
association between immune response and enhanced chemotherapy effect.
Expert Opin Biol Ther 2010;10:983-91.
- Hollingsworth JW, Theriot BS, Li Z, et al. Both hematopoietic-derived and
non-hematopoietic-derived {beta}-arrestin-2 regulates murine allergic
airway disease. Am J Respir Cell Mol Biol 2010;43:269-75.
- Kreisel D, Krupnick AS, Gelman AE, et al. Non-hematopoietic allograft
cells directly activate CD8+ T cells and trigger acute rejection: an
alternative mechanism of allorecognition. Nat Med 2002;8:233-9.
Cite this article as: Lin X, Li W, Lai J, Okazaki M,
Sugimoto S, Yamamoto S, Wang X, Gelman AE,
Kreisel D, Krupnick AS. Five-year update on the
mouse model of orthotopic lung transplantation:
Scientific uses, tricks of the trade, and tips for success.
J Thorac Dis 2012;4(3):247-258. doi: 10.3978/
j.issn.2072-1439.2012.06.02
|