|
Case Report
Management of massive soft tissue defects: The use of INTEGRA®
artificial skin after necrotizing soft tissue infection of the chest
Omar M. Rashid, Masayuki Nagahashi, Kazuaki Takabe
Division of Surgical Oncology, Department of Surgery, Virginia Commonwealth University and Massey Cancer Center, Richmond, Virginia, USA
Kazuaki Takabe, MD, PhD, FACS. Surgical Oncology/VCU, P.O.
Box 980011, West Hospital, 7-402, 1200 East Broad Street Richmond, VA 23298-
0011, USA. Tel: 804-828-9322; Fax: 804-828-4808. Email: ktakabe@vcu.edu.
|
|
Abstract
Necrotizing soft tissue infection, such as necrotizing fasciitis, is a group of highly lethal infections especially when the
chest is involved due to increased risk of pulmonary complications. Because aggressive radical debridement of all poorly
perfused tissue is required, patients frequently suffer from massive skin defects, which often requires autograft skin grafting
or myocutaneous flaps. However, options are limited in patients with limited autograft donor availability, or questionable
underlying wound bed viability, such as in scleroderma. Here, we report the case of a 49 year old female with scleroderma
who suffered from a necrotizing soft tissue infection of the chest extending to her right upper arm, underwent multiple
radical debridements, and reconstruction of the consequent massive chest wall defect with INTEGRA® bilaminar dermal
regeneration template. This approach required a thinner skin graft without flaps, allowed for the inherently diseased
donor site to heal adequately, and avoided major infections and wound complications. This report highlights an important
management option for this challenging disease.
Key words
Chest necrotizing fasciitis; Integra J Thorac Dis 2012;4(3):331-335. DOI: 10.3978/j.issn.2072-1439.2012.05.12
|
|
Introduction
Necrotizing soft tissue infection (NSTI), such as necrotizing
faciitis, is a rapidly progressive and potentially fatal soft tissue
infection that requires prompt, radical, and often multiple surgical
debridements of all necrotic and poorly perfused tissue ( 1, 2).
The mortality rate is high especially when the chest is involved
because such patients are at even greater risk for pulmonary
complications ( 2-4). Progressive skin necrosis may occur
from polymicrobial symbiosis and synergy, infectious spread
or hypotension, which require the patient to undergo further
debridements as often as necessary, which often result in massive
soft tissue defects ( 2-4). Traditionally, reconstruction has been performed with skin
grafts and flaps, as in burn reconstruction ( 2). For large defects
of the chest, because chest wall muscles are often sacrificed
during debridement with difficult wounds to cover, well
vascularized tissue flaps have been traditionally advocated ( 5, 6).
In cases of massive soft tissue defects, autograft reconstruction
may be limited by donor-site availability or questionable
underlying wound bed viability, and may require artificial
alternatives such as INTEGRA ® or Alloderm ® ( 2, 7, 8). INTEGRA®
Dermal Regeneration Template artificial skin is a bilayered
membrane consisting of an inner dermal substitute and an outer
silicone epidermal layer ( 7). This product allows for immediate
tissue coverage to reduce fluid, protein, and electrolyte loss,
protection from microbial invasion, less painful wound care,
and an ultrathin autograft harvest with decreased donor-site
healing times and decreased hypertrophic scarring ( 7). Here, we
report a case of a patient with a large chest soft tissue defect after
multiple debridements for NSTI with reconstruction options
limited by a history of scleroderma, who underwent successful
reconstruction with INTEGRA ®.
|
|
Case report
A 49-year-old female with a history of scleroderma and
hypertension presented to an outside institution with one week
history of worsening swelling, tenderness, and induration of her
right anterior chest, extending to her right upper arm. The patient
had noticed two “small bumps” one week earlier which she
thought was attributable to insect bites. She denied any recent trauma, fever or chills. On presentation at the outside institution,
she was afebrile and subsequently developed hypotension.
Physical examination was consistent with cellulitis of the right
anterior chest inferior to the breast. Laboratory findings were
significant for leukocytosis of 60,700 with 93 segments, an
elevated CPK of 416 and creatinine of 3.5. The patient was
admitted to ICU, but her cellulitis progressively worsened despite
aggressive antimicrobial treatment with Penicillin, Oxycillin, and
Gentamycin. Therefore, extensive debridement was performed
on the following day. Operative findings were consistent with
necrotizing soft tissue infection (NSTI). Cultures from her blood
and wound demonstrated Methicillin Sensitive Staphylococcus
Aureus (MSSA). No anaerobes or other organisms were present.
Antimicrobial coverage was deescalated to Cefazolin on day 4,
and the patient was then transferred to University of California
San Diego Medical Center for further wound management.
On admission, the patient was intubated for pneumonia,
but afebrile with a leukocytosis of 15,300 with 76 segments, no
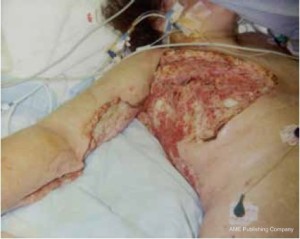
bands. A large skin and soft tissue defect around her right axilla
extending both to anterior of the chest including right medial
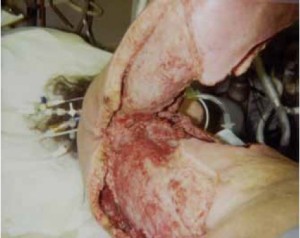
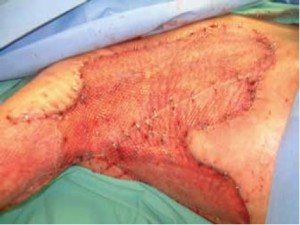
upper arm ( Figure 1), and to posterior of the chest ( Figure 2)
were noted. The underlying muscle and remaining chest wall
were clean with granulation tissue. Initially she was treated
with Penicillin G and Clindamycin ( 9), but then transitioned
to Cefazolin due to leukocytosis increased to 37,600 with 64
segments and 22 bands on day 7 from her presentation. She underwent multiple extensive debridements of the
infected tissue of the chest, torso, and right upper arm under
general anesthesia on days 7, 13, and 18, eventually losing 15%
of her total body surface area, approximately 1,800 cm2 of her
skin. Allograft was applied to the debrided areas and Sulfamylon
solution was applied topically. She was successfully extubated
on day 15, pseudomonas bacteremia and candida urinary tract
infections resolved, and her WBC returned to 10,100. Therefore,
the care was transferred to UCSD Regional Burn Center for
reconstruction of the skin defect. Due to the massive skin defect
extending to her back ( Figure 2), myocutaenous flaps using
latissimus dorsi muscle were deemed impossible to cover all
the defects. Furthermore, given her scleroderma, the donor
skin needed to be as thin as possible to secure adequate donor site
healing. Therefore, the decision was made to utilize INTEGRA ®
Dermal Regeneration Template to cover the exposed tissue to
reduce fluid, protein, and electrolyte loss, minimize microbial
invasion, and allow ultrathin autograft harvest by generation of a
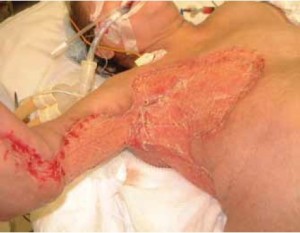
neodermis prior to skin graft. The patient was taken to the operating room for debridement
and placement of INTEGRA ® on day 25 ( Figure 3). INTEGRA ®
was meshed 1:1 prior to placement, and the wound was dressed
with half-strength Sulfamylon solution applied dressing, which
remained intact without purulent drainage for a 10-day period.
On day 38, a small amount of exudate was removed from the
right axilla, which was positive for MSSA. Bactrim was initiated
and small pieces of INTEGRA ® were removed. The remaining
INTEGRA ® in the right upper arm, torso, and chest remained intact
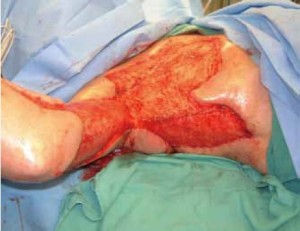
with good wound bed formation. The outer layer of INTEGRA ® was
removed on day 46 when good neodermis formation was confirmed
( Figure 4). Split-thickness skin graft, meshed at 1:1.5, was
performed by harvesting several pieces of autograft set at 0.009
inches with the Zimmer dermatome from her bilateral anterior
thighs, with Betaglucan dressing applied to the donor site ( Figure 5). Her postoperative course was free from any complication at
both her donor and grafted sites, and she was discharged on day 56. Both the grafts and the bilateral anterior thigh donor sites
healed very well, requiring only moisturizer on discharge.
|
|
Discussion
Necrotizing soft tissue infection (NSTI) is a critically serious
infection of the subcutaneous tissue and fascia which requires
timely diagnosis and aggressive, wide, radical debridements of
all necrotic and poorly perfused tissue to optimize outcomes
( 2, 10-12). The mortality approaches 70% in reported series,
and management is especially challenging when the chest is
involved, where mortality is often greater than 89% ( 2-4, 13, 14).
Hemodynamic instability usually persists postoperatively, and
progressive skin necrosis may occur from infectious spread,
hypotension, and massive fluid and protein losses. Affected
patients must return for further debridements as often as
necessary, further exacerbating tissue loss often with wound
complications, prolonged sepsis, and poor nutrition ( 1, 2, 11-15).
There is evidence that aggressive, early surgical debridement
in combination with appropriate antimicrobial therapy,
resuscitation, nutritional support, and improved critical
and wound care limit the extent of soft tissue loss ( 2, 16, 17).
However, even in such optimal circumstances, soft tissue
defects are often massive and pose a challenge to clinicians ( 2).
Once all diseased tissue has been debrided and the patient has
been stabilized, soft tissue reconstruction can be considered.
Traditionally, reconstruction has been performed with skin grafts
and myocutanesous flaps when primary closure is not possible,
as in burn reconstruction. In cases with excessively large
amounts of soft tissue involvement (>25% Body Surface Area),
autograft reconstruction may be restricted by limited donor-site
availability or questionable underlying wound bed viability, such
as a history of scleroderma in our patient ( 2). Scleroderma is a connective tissue disease whose principal features
include extensive fibrosis, vascular alterations, and autoantibodies
against self-antigens which affect wound healing ( 18). The
progression of the disease is from early microvascular damage,
mononuclear-cell infiltrates, and slowly developing fibrosis to
densely packed collagen in the dermis, cell loss and atrophy ( 18).
Despite the progressive loss of blood vessels and high serum
levels of vascular endothelial growth factor and other angiogenic
factors, there is a defect in vasculogenesis ( 18-21). There is a
gradual replacement of the blood vessels and normal architecture
with fibrosis and a mixture of collagens, proteoglycans and elastic
fibers ( 18). In addition, fibroblasts which coordinate collagen
and extracellular matrix component production, deposition, and remodeling overexpress cytokines and reactive oxygen
species which further the progression of fibrosis and alteration
of normal wound healing ( 18). The process of fibrosis and
impaired vasculogenesis are further promoted by autoantibodies
which inhibit factors that would otherwise promote normal
wound healing and angiogenesis, such as PDGF ( 18). Because
of these features of scleroderma, the challenge of skin defect
reconstruction in our patient was that the donor sites of any
autograft thick enough to fill the defect, as well as the recipient
site itself, would be at high risk of wound complications. To
utilize thinner pieces of autograft, a means to develop dermal
regeneration was required. Since the skin defect was massive
and the wound was not actively infected, we chose to apply
INTEGRA ® to fill the defect for the following reasons. INTEGRA® is a bilayered membrane system, consisting of an
inner dermal substitute layer and a temporary outer epidermal
substance layer. The inner layer is composed of a 3-demensional
matrix of cross-linked bovine tendon collagen plus a
glycosaminoglycan, and an outer layer is composed of silicone.
After application of INTEGRA® the patient’s native fibroblasts,
macrophages, and lymphocytes infiltrate, and new capillary
growth occur into the matrix of the inner layer. The inner layer
becomes degraded, and an endogenous collagen matrix is
deposited by the patient’s own fibroblasts, forming a “neodermis”.
This process can take place without creating any new wounds in
the patient because no donor sites are created. Once engraftment
is complete, which usually occurs approximately 2 weeks after
application, the outer silicone layer needs to be removed, and an
epidermal autograft must placed over the “neodermis”.
The advantages of this product are the following: (I)
immediate tissue coverage to reduce fluid, protein, and
electrolyte loss from the grafted surfaces; (II) protection of the
wound from microorganism invasion; (III) less painful wound
care after grafting; (IV) thinner autografts with decreased donorsite
healing time; and (V) less hypertrophic scarring ( 22). With
these advantages, it was initially developed for treating large burn
wounds ( 22). A contraindication to the use of INTEGRA ® is that
if there is an active secondary wound infection, “neodermis” will
not form. In such circumstances allografts are desirable ( 23),
which would have been the case for our patient if her wound had
not stabilized. Because infection was a concern prior to applying
INTEGRA ® and to avoid infection of the INTEGRA ®, we meshed
it 1:1 prior to placement. In our anecdotal experience, infection
decreased from 55% to 15% with mesh. We have also tried “Piecrusting”
the INTEGRA ®, which did not decrease the infection
rate. The result was that our patient successfully underwent
neodermis formation requiring a thin skin graft just for epidermal
coverage without any donor wound site complications. In summary, NSTI of the chest wall poses a formidable
challenge to clinicians, not only in the appropriate early
diagnosis and aggressive management of the disease, but
also reconstruction of the soft tissue defect after appropriate
management of the initial insult. INTEGRA® artificial skin
offered the advantages of neodermis formation without the
risks of thick autograft harvesting in a patient with a massive
defect without major complications, despite a patient history of
scleroderma. Therefore, our case highlights an important clinical
option to consider for patients with massive chest wall soft tissue
defects in whom full thickness skin grafts and myocutaenous
flaps would be high risk.
|
|
Acknowledgements
Kazuaki Takabe is supported by United States National Institute
of Health (R01CA160688, K12HD055881, R01CA154314, and
R01DK057543) and Susan G. Komen for the Cure (Investigator
Initiated Research Grant (222224) and Career Catalyst Research
Grant KG090510). Masayuki Nagahashi is a Japan Society
for the Promotion of Science ( JSPS) Postdoctoral Fellow for
Research Abroad.
Disclosure: The authors declare no conflict of interest.
|
|
References
- Drake DB, Woods JA, Bill TJ, et al. Magnetic resonance imaging in the early
diagnosis of group A beta streptococcal necrotizing fasciitis: a case report. J
Emerg Med 1998;16:403-7.
- Edlich RF, Winters KL, Woodard CR, et al. Massive soft tissue infections:
necrotizing fasciitis and purpura fulminans. J Long Term Eff Med Implants
2005;15:57-65.
- Urschel JD. Necrotizing soft tissue infections. Postgrad Med J 1999;75:645-9.
- Urschel JD, Takita H, Antkowiak JG. Necrotizing soft tissue infections of
the chest wall. Ann Thorac Surg 1997;64:276-9.
- Cohen M, Ramasastry SS. Reconstruction of complex chest wall defects.
Am J Surg 1996;172:35-40.
- Quirk WF Jr, Sternbach G. Joseph Jones: infection with flesh eating
bacteria. J Emerg Med 1996;14:747-53.
- Frame JD, Still J, Lakhel-LeCoadou A, et al. Use of dermal regeneration
template in contracture release procedures: a multicenter evaluation. Plast
Reconstr Surg 2004;113:1330-8.
- Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in
the management of full-thickness burns. Burns 1995;21:243-8.
- Seal DV. Necrotizing fasciitis. Curr Opin Infect Dis 2001;14:127-32.
- Lewis RT. Soft tissue infections. World J Surg 1998;22:146-51.
- Lille ST, Sato TT, Engrav LH, et al. Necrotizing soft tissue infections:
obstacles in diagnosis. J Am Coll Surg 1996;182:7-11.
- Meleney FL. Reminiscences of forty-five years dealing with surgical
infections. Rev Surg 1964;21:307-26.
- Chelsom J, Halstensen A, Haga T, et al. Necrotising fasciitis due to group
A streptococci in western Norway: incidence and clinical features. Lancet
1994;344:1111-5.
- Wang KC, Shih CH. Necrotizing fasciitis of the extremities. J Trauma 1992;32:179-82.
- Sudarsky LA, Laschinger JC, Coppa GF, et al. Improved results from a
standardized approach in treating patients with necrotizing fasciitis. Ann
Surg 1987;206:661-5.
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis.
Arch Intern Med 1985;145:1020-3.
- Kaufman JL. Clinical problem-solving: necrotizing fasciitis. N Engl J Med
1994;331:279; author reply 280.
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med
2009;360:1989-2003.
- Cipriani P, Guiducci S, Miniati I, et al. Impairment of endothelial cell
differentiation from bone marrow-derived mesenchymal stem cells:
new insight into the pathogenesis of systemic sclerosis. Arthritis Rheum
2007;56:1994-2004.
- Fleming JN, Nash RA, McLeod DO, et al. Capillary regeneration
in scleroderma: stem cell therapy reverses phenotype? PLoS One
2008;3:e1452.
- Kuwana M, Okazaki Y, Yasuoka H, et al. Defective vasculogenesis in
systemic sclerosis. Lancet 2004;364:603-10.
- Burke JF, Yannas IV, Quinby WC Jr, et al. Successful use of a physiologically
acceptable artificial skin in the treatment of extensive burn injury. Ann Surg
1981;194:413-28.
- Chasan PE, Hansbrough JF, Cooper ML. Management of cutaneous
manifestations of extensive purpura fulminans in a burn unit. J Burn Care
Rehabil 1992;13:410-3.
Cite this article as: Rashid OM, Nagahashi M, Takabe K. Management
of massive soft tissue defects: The use of INTEGRA® artificial skin after
necrotizing soft tissue infection of the chest. J Thorac Dis 2012;4(3):331-
335. doi: 10.3978/j.issn.2072-1439.2012.05.12
|